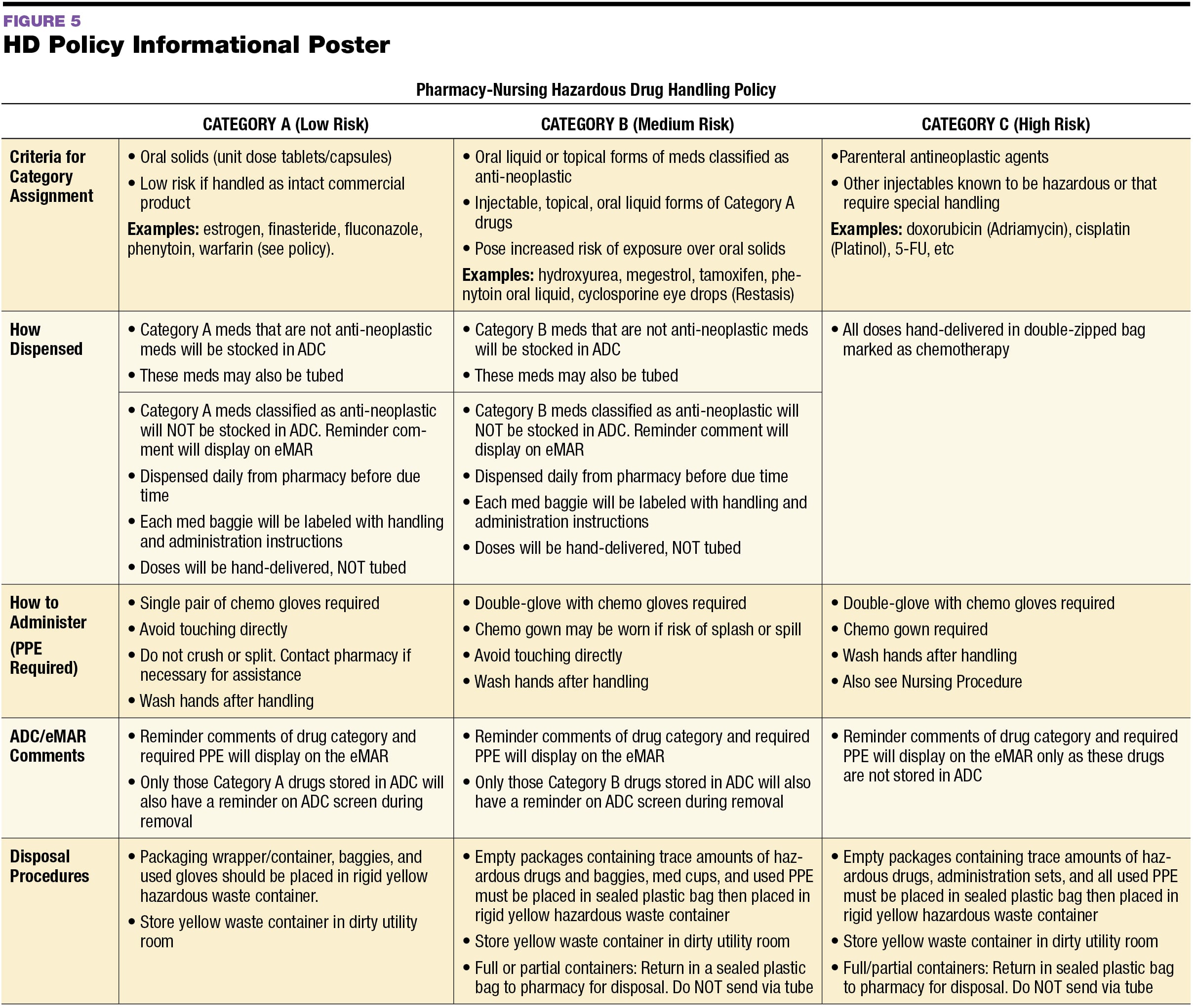
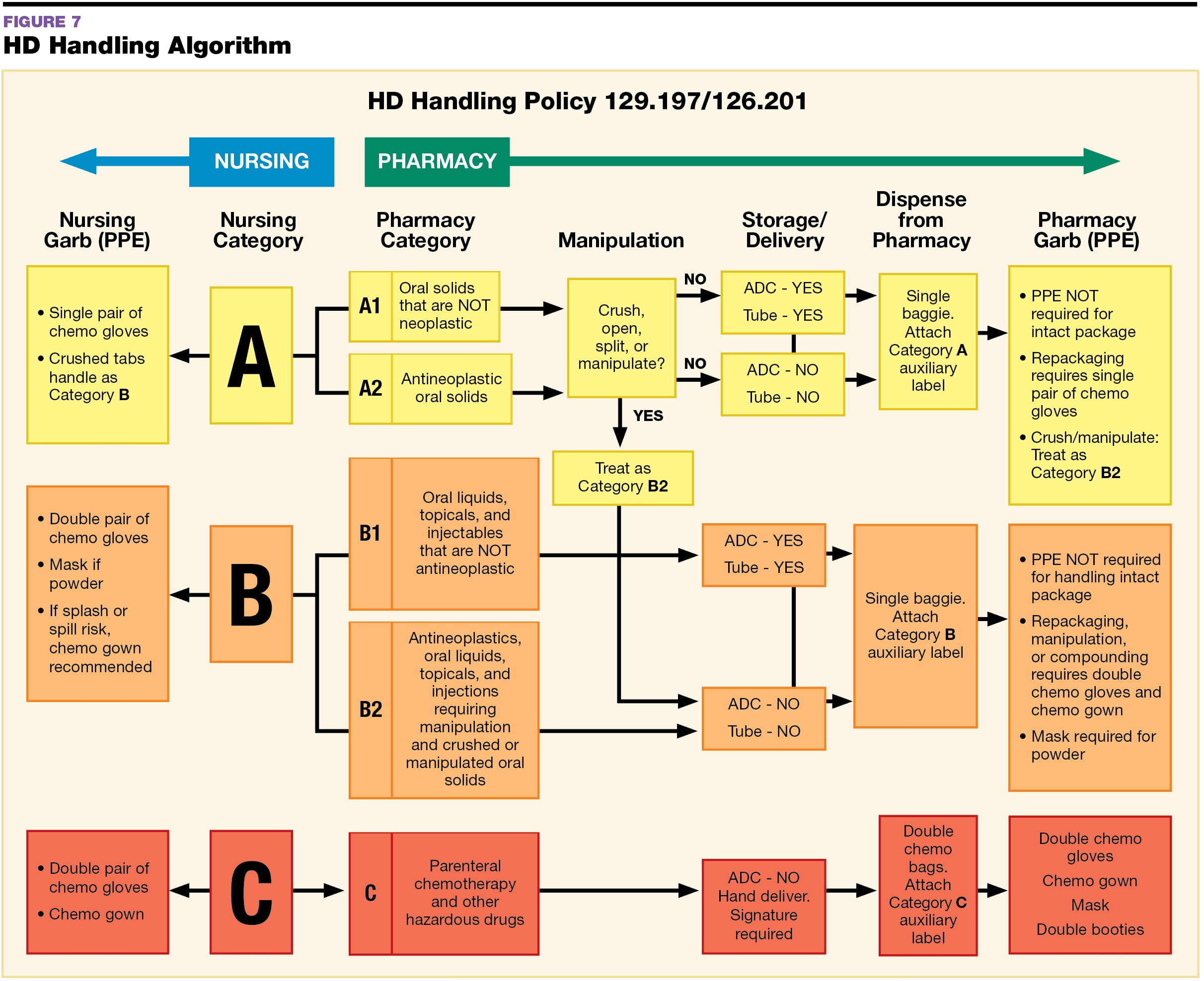
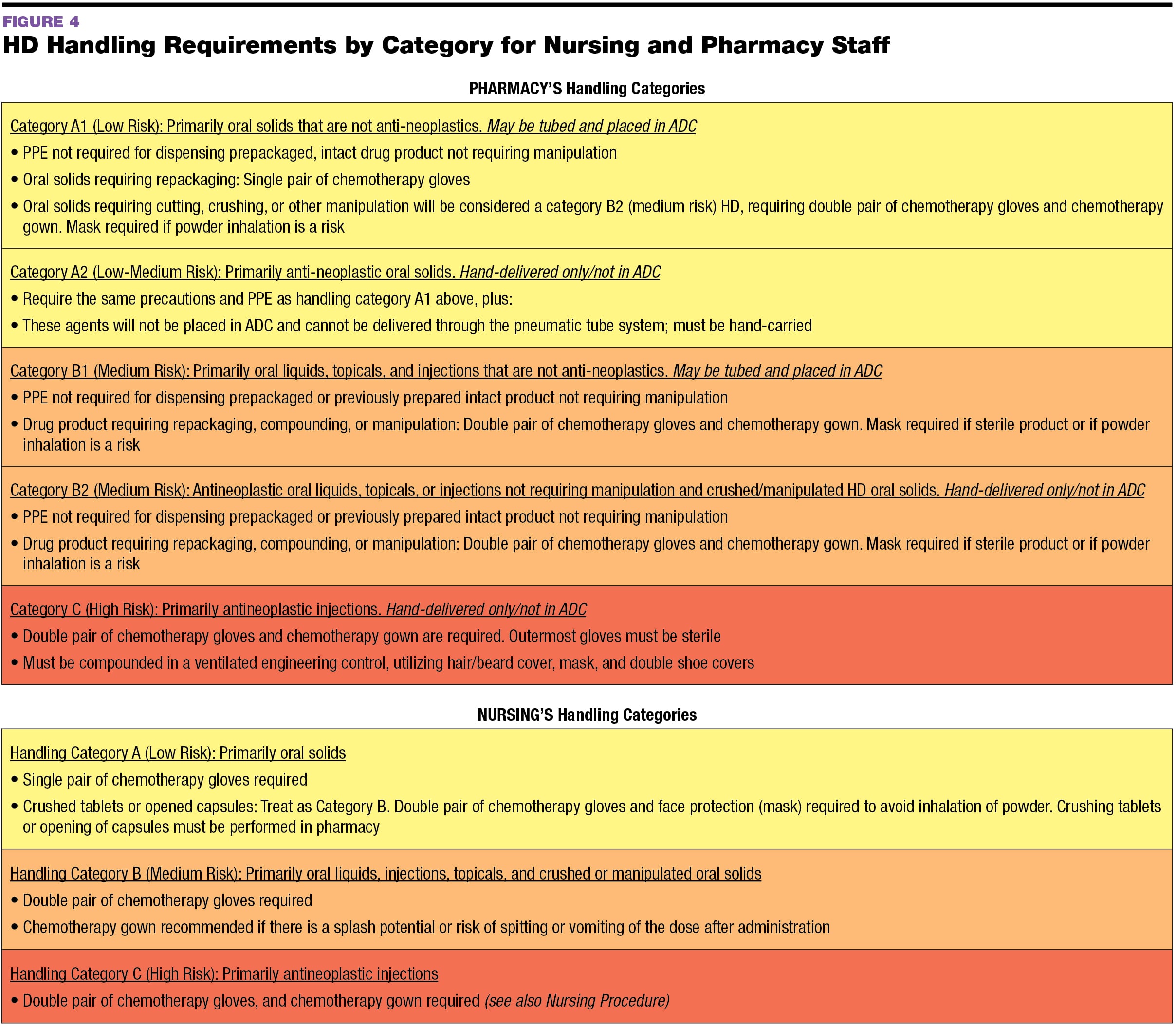
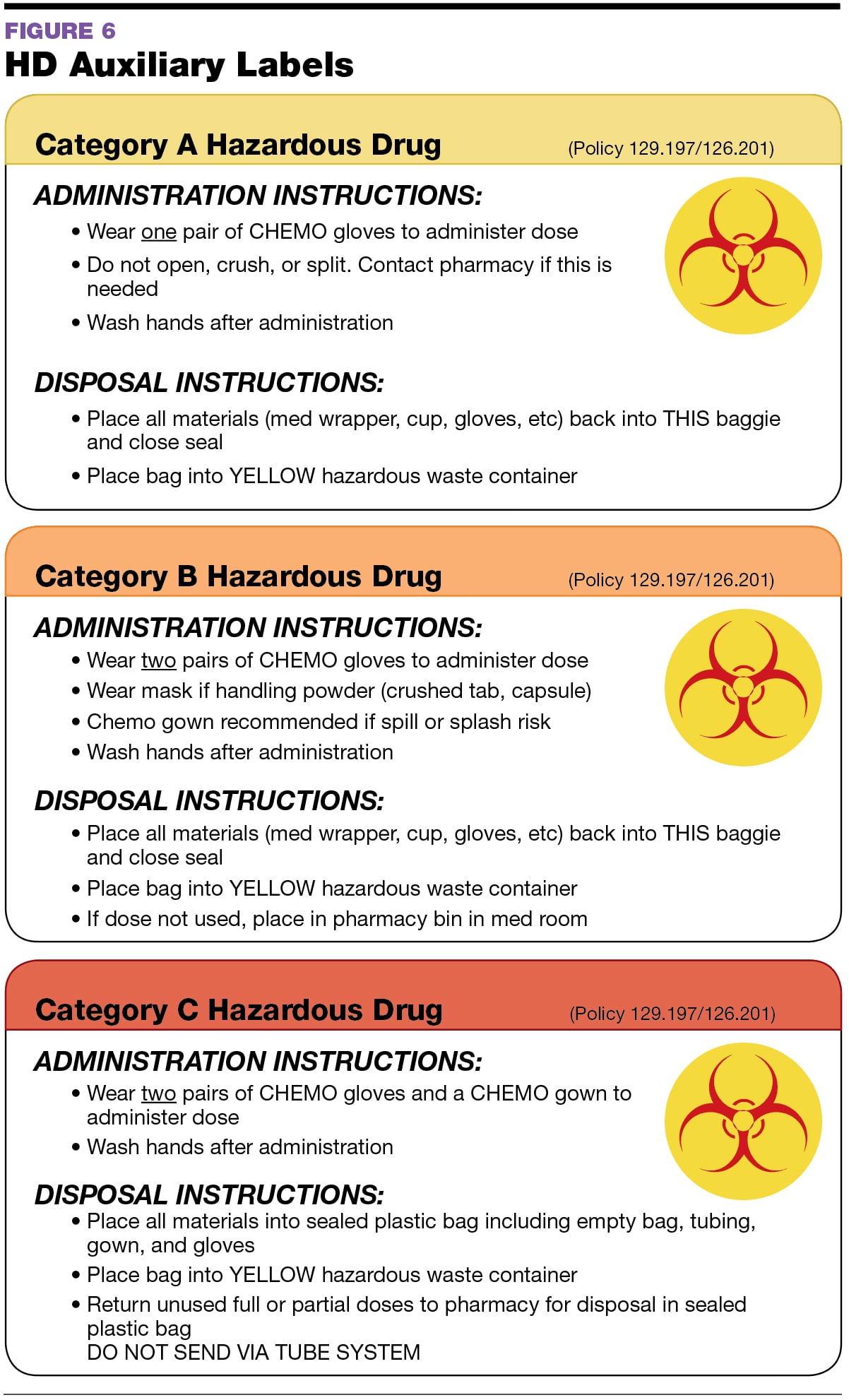
Usp 800 Policy And Procedures Retail Pharmacy Template
Usp 800 Policy And Procedures Retail Pharmacy Template - Usp <<strong>800</strong>> can be accessed via the usp. States may place <<strong>800</strong>> into state regulations. Conducted in the second quarter of 2021, pp&p ’s 4 th annual survey of usp <<strong>800</strong>> compliance yielded a confidence. Complete a risk assessment and. Web templates are fully customizable and enable facilities to create electronic policy manuals, detailing everything from floor plans and diagrams for how drugs move. In addition, it is a required policy for. Web ncpa has developed a blank usp <800> risk assessment template, and a sample template for testosterone, to help you create your own risk assessments for. Web usp general chapter <<strong>800</strong>> provides standards for safe handling of hazardous drugs to minimize the risk of exposure to healthcare personnel, patients and the environment. <<strong>800</strong>> will become federally enforceable on july 1, 2018. Web some state boards of pharmacy are now requiring pharmacies to have a usp800 compliance plan. •scope of <<strong>800</strong>> •<<strong>800</strong>> compliance •personnel •facility •community pharmacy. <<strong>800</strong>> will become federally enforceable on july 1, 2018. But nonetheless, first things first,. In 2019, new guidelines on handling hazardous. Web below are steps that your pharmacy can take to meet the applicable compliance team accreditation quality standard: Usp <<strong>800</strong>> can be accessed via the usp. Starting this year, most pbms require this policy for recredentialing. Web some state boards of pharmacy are now requiring pharmacies to have a usp800 compliance plan. Web usp general chapter <<strong>800</strong>> provides standards for safe handling of hazardous drugs to minimize the risk of exposure to healthcare personnel, patients and the environment.. States may place <<strong>800</strong>> into state regulations. Web community retail pharmacy policies & procedures includes controlled substances protocols & usp 800 hazardous drug control program & anti kickback policies. Web usp general chapter <<strong>800</strong>> provides standards for safe handling of hazardous drugs to minimize the risk of exposure to healthcare personnel, patients and the environment. Web templates are fully customizable. But, you know, i think pharmacists have a good idea of which ones those are. Web usp general chapter <<strong>800</strong>> provides standards for safe handling of hazardous drugs to minimize the risk of exposure to healthcare personnel, patients and the environment. Web below are steps that your pharmacy can take to meet the applicable compliance team accreditation quality standard: Web. Web best practice guidelines on handling hazardous drugs under usp general chapter <<strong>800</strong>>. Complete a risk assessment and. Chapter <<strong>800</strong>> applies to all health care personnel who handle hd preparation and all entities that store, prepare, transport or administer hds (e.g., pharmacies, hospitals. Web usp general chapter <<strong>800</strong>> provides standards for safe handling of hazardous drugs to minimize the risk. •scope of <<strong>800</strong>> •<<strong>800</strong>> compliance •personnel •facility •community pharmacy. Web and, honestly, some are more hazardous than others. Web some state boards of pharmacy are now requiring pharmacies to have a usp800 compliance plan. Web usp general chapter <<strong>800</strong>> provides standards for safe handling of hazardous drugs to minimize the risk of exposure to healthcare personnel, patients and the environment.. Web and, honestly, some are more hazardous than others. Web templates are fully customizable and enable facilities to create electronic policy manuals, detailing everything from floor plans and diagrams for how drugs move. Web below are steps that your pharmacy can take to meet the applicable compliance team accreditation quality standard: Complete a risk assessment and. States may place <<strong>800</strong>>. Web board staff have created helpful educational information that addresses identification of hazardous drugs, instructions on conducting an assessment of risk, and templates for. Web the usp <<strong>800</strong>> policies and procedures templates. Usp <<strong>800</strong>> can be accessed via the usp. States may place <<strong>800</strong>> into state regulations. But nonetheless, first things first,. Web templates are fully customizable and enable facilities to create electronic policy manuals, detailing everything from floor plans and diagrams for how drugs move. Chapter <<strong>800</strong>> applies to all health care personnel who handle hd preparation and all entities that store, prepare, transport or administer hds (e.g., pharmacies, hospitals. Bula helps you prepare to maintain compliance by offering the following. Conducted in the second quarter of 2021, pp&p ’s 4 th annual survey of usp <<strong>800</strong>> compliance yielded a confidence. The future intent is for usp <<strong>800</strong>>. Starting this year, most pbms require this policy for recredentialing. Complete a risk assessment and. Web usp <<strong>800</strong>> applies to handling of hazardous drugs (hds) where there is a risk of exposure to. <<strong>800</strong>> will become federally enforceable on july 1, 2018. Web current efforts are focused on implementing the required elements of usp 800 designed to ensure policies, procedures, work practices and engineering controls (e.g.,. But, you know, i think pharmacists have a good idea of which ones those are. Bula helps you prepare to maintain compliance by offering the following three usp 800 policies and procedures. States may place <<strong>800</strong>> into state regulations. Starting this year, most pbms require this policy for recredentialing. Web and, honestly, some are more hazardous than others. Web some state boards of pharmacy are now requiring pharmacies to have a usp800 compliance plan. Complete a risk assessment and. In addition, it is a required policy for. Chapter <<strong>800</strong>> applies to all health care personnel who handle hd preparation and all entities that store, prepare, transport or administer hds (e.g., pharmacies, hospitals. Web community retail pharmacy policies & procedures includes controlled substances protocols & usp 800 hazardous drug control program & anti kickback policies. Web below are steps that your pharmacy can take to meet the applicable compliance team accreditation quality standard: Conducted in the second quarter of 2021, pp&p ’s 4 th annual survey of usp <<strong>800</strong>> compliance yielded a confidence. Web the usp <<strong>800</strong>> policies and procedures templates. Web best practice guidelines on handling hazardous drugs under usp general chapter <<strong>800</strong>>. Usp <<strong>800</strong>> can be accessed via the usp. Web policy usp 800 is available for download. In 2019, new guidelines on handling hazardous. Web ncpa has developed a blank usp <800> risk assessment template, and a sample template for testosterone, to help you create your own risk assessments for.A Comprehensive Approach to USP Compliance November 2017 Pharmacy
Usp 800 Sop Template Master of Documents
USP 800 Compliance for Compounding Pharmacies & Laboratories
A Comprehensive Approach to USP Compliance November 2017 Pharmacy
A Comprehensive Approach to USP Compliance November 2017 Pharmacy
USP800 Pharmacy Compliance PRS Pharmacy Services
A Comprehensive Approach to USP Compliance November 2017 Pharmacy
Prepare Pharmacy and Nursing for USP July 2015 Pharmacy Purchasing
USP 800 PDF Download Form Medline
Compliance with USP 800 — RxPolicy
Related Post: