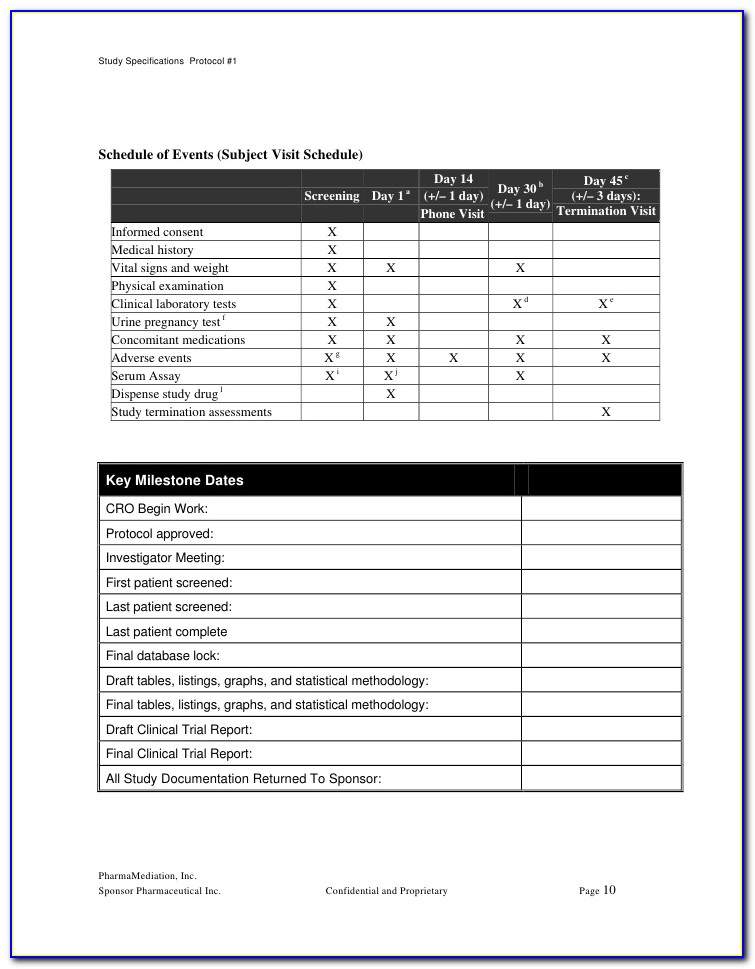
Trial Master File Checklist Template
Trial Master File Checklist Template - Web trial master file checklist template. The tmf structure is commonly broken down into the following zones: Welcome to global health trials' tools and templates library. The tmf plan template subgroup of the tmf reference model project is pleased to announce that the tmf plan template version. This station is part of the ‘trial planning phase’ group of stations. Budget monitoring tool with example data. Not all documents listed below will be applicable to all trials. Web tmf plan template now available. Have the attorney meet with the client in preparation for. Select popular legal forms & packages of any category. A document management device by tmf document types, trial master file checklist model,. Not all documents listed below will be applicable to all trials. Web tmf plan template now available. Web mastercontrol tmf checklist template. Web this checklist should be used as a tool to identify which essential documents should be filed in trial master files (tmfs) and investigator site. Web trial master file (tmf) frequently asked questions. Web details for trial master file checklist. The tmp template is designed to streamline the. Click on link to provide. Web tmf plan template now available. Web tmf plan template now available. Welcome to global health trials' tools and templates library. This station is part of the ‘trial planning phase’ group of stations. Select popular legal forms & packages of any category. The tmf structure is commonly broken down into the following zones: Web mastercontrol tmf checklist template. The tmf structure is commonly broken down into the following zones: The tmp template is designed to streamline the. Web this checklist should be used as a tool to identify which essential documents should be filed in trial master files (tmfs) and investigator site files (isfs), and also. Have the attorney meet with the client. Budget monitoring tool with example data. Not all documents listed below will be applicable to all trials. Web tmf plan template now available. Web the trial master file is a legal requirement that is relevant to all trials. Web in homage to the new year, we’ve put together a trial master file checklist of 20 new year’s resolutions to unleash. Web trial master file reference model v3.2.1. Web trial master file checklist template. Jrmo investigator site file checklist template v0.1 page 1 of 4. Web mastercontrol tmf checklist template. The templates below have been shared by other groups, and are free to use and adapt for your. Web clinical study report template. The templates below have been shared by other groups, and are free to use and adapt for your. The tmf reference model defines standard contents, structure, terminology and metadata for the trial master file, essential. Welcome to global health trials' tools and templates library. Select popular legal forms & packages of any category. Web an agency of the european union. Web trial master file checklist (ctimp) this checklist should be used as a guide only. The tmf plan template subgroup of the tmf reference model project is pleased to announce that the tmf plan template version. Jrmo investigator site file checklist template v0.1 page 1 of 4. A trial master file (tmf) should. Web in homage to the new year, we’ve put together a trial master file checklist of 20 new year’s resolutions to unleash your team's full potential (as 2,020 items would have been. Not all documents listed below will be applicable to all trials. Web this checklist should be used as a tool to identify which essential documents should be filed. Web mastercontrol tmf checklist template. The tmf reference model defines standard contents, structure, terminology and metadata for the trial master file, essential. Not all documents listed below will be applicable to all trials. Guideline on the content, management and archiving of the clinical trial master file (paper and/or electronic) 1. Web this checklist should be used as a tool to. Why use this trial master file to do checklist? The protocol summary should contain the study objectives, design overview, inclusion/exclusion criteria, and patient. Guideline on the content, management and archiving of the clinical trial master file (paper and/or electronic) 1. Web trial master file checklist (ctimp) this checklist should be used as a guide only. The tmf reference model defines standard contents, structure, terminology and metadata for the trial master file, essential. Welcome to global health trials' tools and templates library. Web an agency of the european union. Web tmf plan template now available. Select popular legal forms & packages of any category. The tmp template is designed to streamline the. A trial master file (tmf) should be set up. Web this checklist should be used as a tool to identify which essential documents should be filed in trial master files (tmfs) and investigator site files (isfs), and also. Web details for trial master file checklist. Jrmo investigator site file checklist template v0.1 page 1 of 4. The templates below have been shared by other groups, and are free to use and adapt for your. The tmf plan template subgroup of the tmf reference model project is pleased to announce that the tmf plan template version. Web the trial master file is a legal requirement that is relevant to all trials. Not all documents listed below will be applicable to all trials. Web mastercontrol offers a complimentary downloadable trial master file template above in excel spreadsheet format. This station is part of the ‘trial planning phase’ group of stations.Clinical Trial Protocol Synopsis Template
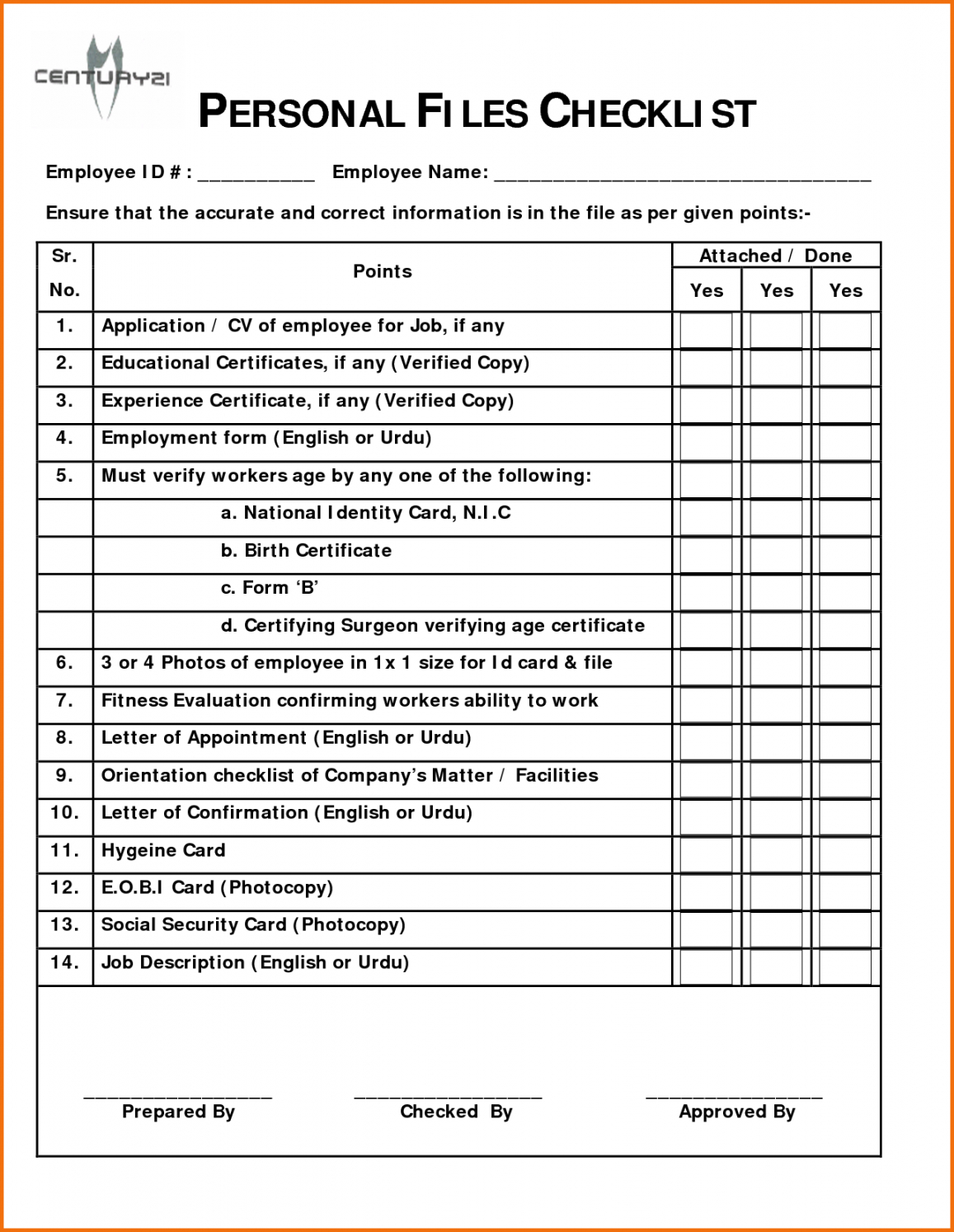
Employee Personnel File Checklist Template
TrialMasterFile Checklist by Pharma Student Issuu
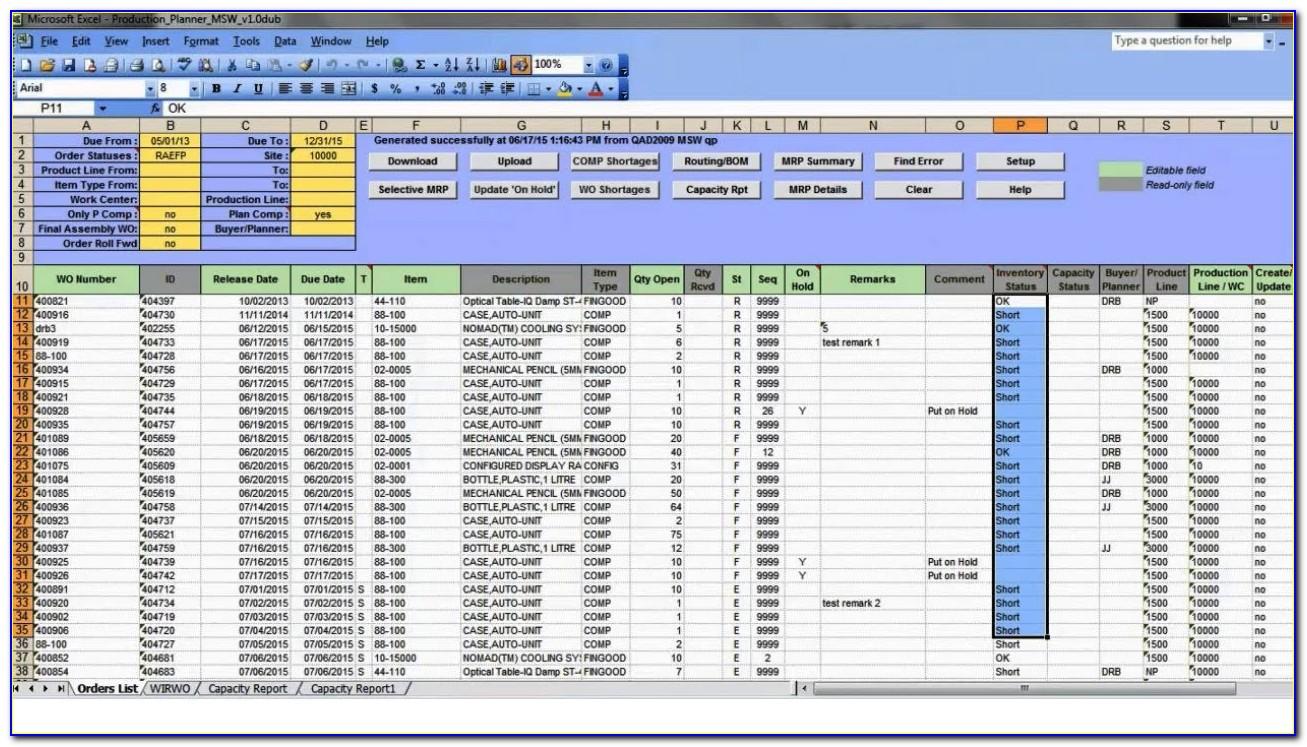
Master Production Schedule Template Excel
Audit Plan Template For Clinical Trials Cards Design Templates
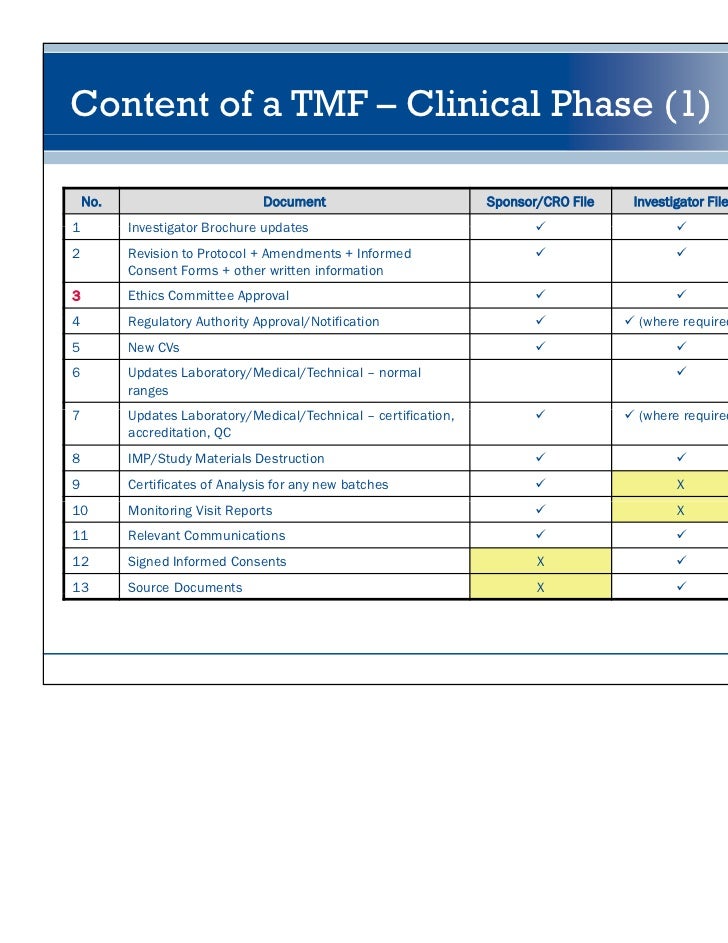
Essential documents and_managing_trial_files
TrialMasterFile Checklist [PDF Document]
Personnel File Checklist Template
Clinical Trial Checklist Clinical Trial Healthcare Quality
Trial Master File PDF Audit Business
Related Post:


![TrialMasterFile Checklist [PDF Document]](https://cdn.vdocuments.site/img/1200x630/reader024/reader/2020123115/568c49221a28ab491692fb56/r-1.jpg?t=1616114390)


