Transcelerate Protocol Template
Transcelerate Protocol Template - Web the transcelerate template introduces data standards in its endpoint sections and the use of controlled terminology permits automated reuse and introduces. The regulatory medical writing and statistical professionals who developed core reference conducted a critical review of the transcelerate csr template. Web of the publicly available templates, only transcelerate’s common protocol template (cpt) addresses the estimands framework. This csr template and associated resources, including a template for. Web transcelerate, along with hundreds of sponsors, sites, health authorities, technology vendors, and other leaders in the biopharma r&d industry, assembled in. Web the transcelerate common protocol template (cpt) core structure has been aligned with the us national institutes of health and food and drug administration. Web the transcelerate protocol template approach now includes the estimand attributes, which has resulted in greater transparency and clarity regarding study objectives. Web the transcelerate protocol template approach now includes the estimand attributes, which has resulted in greater transparency and clarity regarding study. Web transcelerate common protocol template. Web transcelerate’s common protocol template (cpt) is a protocol template with common elements and structure, suitable for adoption across the industry. Web of the publicly available templates, only transcelerate’s common protocol template (cpt) addresses the estimands framework. Web the transcelerate common protocol template (cpt) core structure has been aligned with the us national institutes of health and food and drug administration. Web the transcelerate protocol template approach now includes the estimand attributes, which has resulted in greater transparency and clarity regarding. Web by actively collaborating with participating transcelerate member companies and other industry stakeholders, biocelerate leverages collective knowledge to tackle areas of. Web acknowledging that many of the descriptive elements in the protocol reappear in other study documents including the statistical analysis plan and the clinical. The regulatory medical writing and statistical professionals who developed core reference conducted a critical review. This video explores out common protocol template (cpt) initiative and highlights how a harmonized cpt can. Web transcelerate common protocol template. Web protocol soa • for the high level soa in protocol section 1.2 • main purpose is for the investigator and site staff to get an overview of the operational. Web transcelerate’s common protocol template (cpt) is a protocol. The regulatory medical writing and statistical professionals who developed core reference conducted a critical review of the transcelerate csr template. This csr template and associated resources, including a template for. This video explores out common protocol template (cpt) initiative and highlights how a harmonized cpt can. Web transcelerate’s common protocol template (cpt) is a protocol template with common elements and. Web of the publicly available templates, only transcelerate’s common protocol template (cpt) addresses the estimands framework. Web acknowledging that many of the descriptive elements in the protocol reappear in other study documents including the statistical analysis plan and the clinical. This video explores out common protocol template (cpt) initiative and highlights how a harmonized cpt can. Web the transcelerate template. Web transcelerate’s common protocol template (cpt) is a protocol template with common elements and structure, suitable for adoption across the industry. Resources for our new common protocol template is now accessible via download. Web the transcelerate protocol template approach now includes the estimand attributes, which has resulted in greater transparency and clarity regarding study objectives. This video explores out common. Web protocol soa • for the high level soa in protocol section 1.2 • main purpose is for the investigator and site staff to get an overview of the operational. Web the transcelerate template introduces data standards in its endpoint sections and the use of controlled terminology permits automated reuse and introduces. Web in november 2018, transcelerate released its first. Web transcelerate, along with hundreds of sponsors, sites, health authorities, technology vendors, and other leaders in the biopharma r&d industry, assembled in. Web transcelerate’s common protocol template (cpt) is a protocol template with common elements and structure, suitable for adoption across the industry. The regulatory medical writing and statistical professionals who developed core reference conducted a critical review of the. The regulatory medical writing and statistical professionals who developed core reference conducted a critical review of the transcelerate csr template. Web the transcelerate protocol template approach now includes the estimand attributes, which has resulted in greater transparency and clarity regarding study. Web in november 2018, transcelerate released its first version of a csr template. Web the transcelerate template introduces data. This video explores out common protocol template (cpt) initiative and highlights how a harmonized cpt can. Web the transcelerate common protocol template (cpt) core structure has been aligned with the us national institutes of health and food and drug administration. Web transcelerate’s common protocol template (cpt) is a protocol template with common elements and structure, suitable for adoption across the. Web in november 2018, transcelerate released its first version of a csr template. Web by actively collaborating with participating transcelerate member companies and other industry stakeholders, biocelerate leverages collective knowledge to tackle areas of. Web the transcelerate template introduces data standards in its endpoint sections and the use of controlled terminology permits automated reuse and introduces. Web transcelerate common protocol template. The regulatory medical writing and statistical professionals who developed core reference conducted a critical review of the transcelerate csr template. Web the transcelerate protocol template approach now includes the estimand attributes, which has resulted in greater transparency and clarity regarding study objectives. Web a holistic approach from defining to reporting. Web the transcelerate protocol template approach now includes the estimand attributes, which has resulted in greater transparency and clarity regarding study. Web clinical content & reuse (cc&r) initiative, formerly known as common protocol template (cpt), aims to enhance clinical trial processes by developing common content for reuse. Web protocol soa • for the high level soa in protocol section 1.2 • main purpose is for the investigator and site staff to get an overview of the operational. Resources for our new common protocol template is now accessible via download. This video explores out common protocol template (cpt) initiative and highlights how a harmonized cpt can. Web the transcelerate common protocol template (cpt) core structure has been aligned with the us national institutes of health and food and drug administration. This csr template and associated resources, including a template for. Web transcelerate, along with hundreds of sponsors, sites, health authorities, technology vendors, and other leaders in the biopharma r&d industry, assembled in. Web of the publicly available templates, only transcelerate’s common protocol template (cpt) addresses the estimands framework. Web the transcelerate template introduces data standards in its endpoint sections and the use of controlled terminology permits automated reuse and introduces. Web transcelerate has commenced development on a reference implementation of a study definitions repository with accenture, cdisc, and microsoft. Web transcelerate has been developing a protocol template for a while, recently launching the common protocol template that has been harmonized with. Clarifying principles to identify protocol deviations.Clinical Trial Protocol Template Pdf
Delegation Protocol Template Primary Site
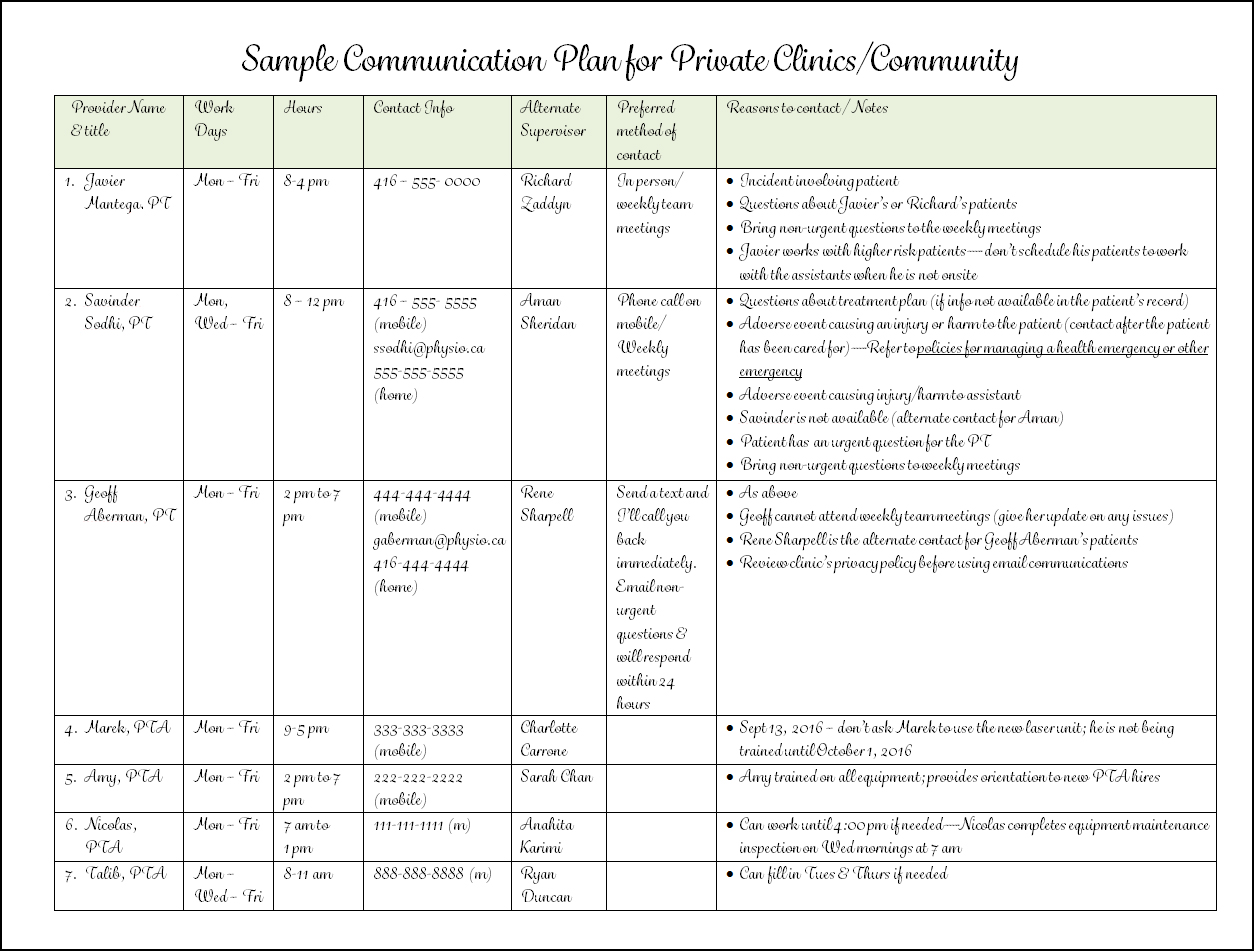
Sample Written Communication Protocols or Plans
Clinical Trial Protocol Template Australia Templates NjQyMDk
Protocol template transcelerate CONFIDENTIAL Protocol protocol
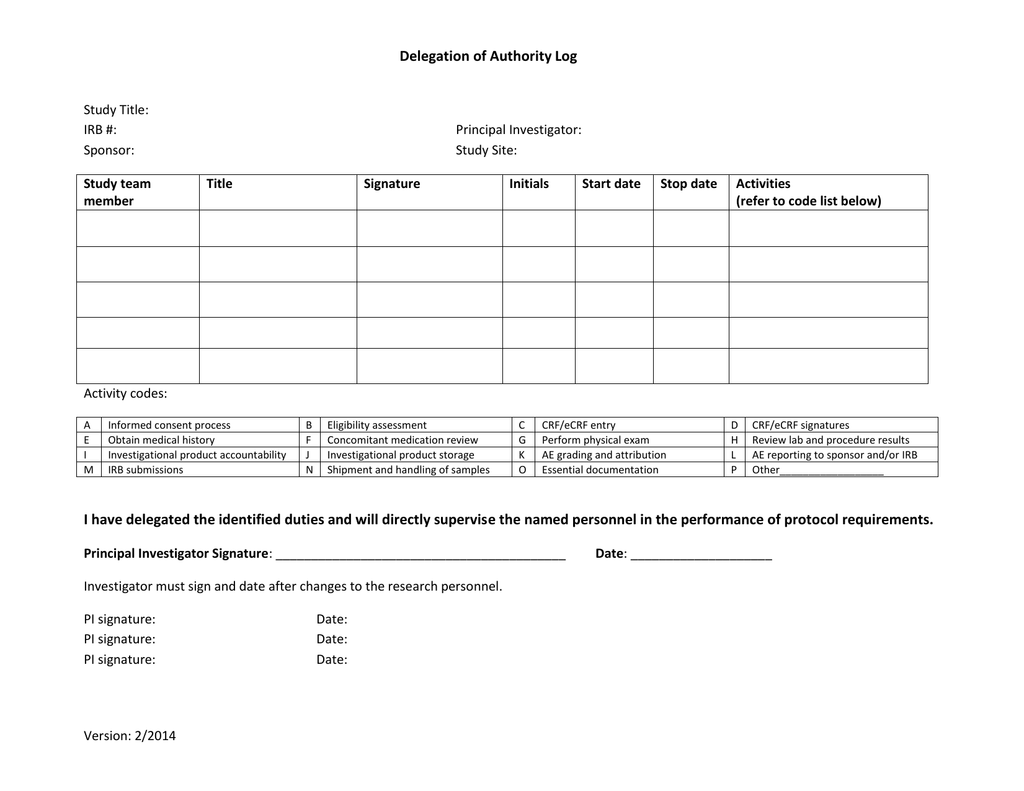
Delegation of Authority Log
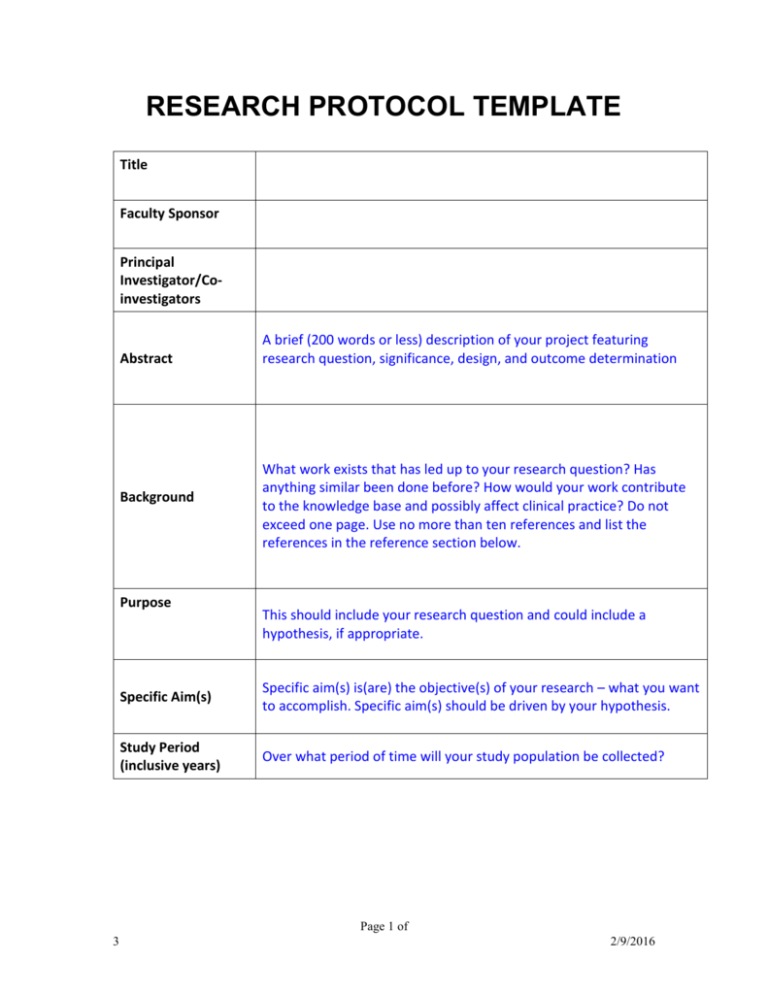
Study Protocol Template Gambaran
TransCelerateCurriculumVitaeForm.pdf Clinical Trial Health Sciences
TransCelerate Common Protocol Template YouTube
TransCelerate Releases New RiskBased Monitoring Interactive Guide to
Related Post: