Strategy For Regulatory Compliance Mdr Template
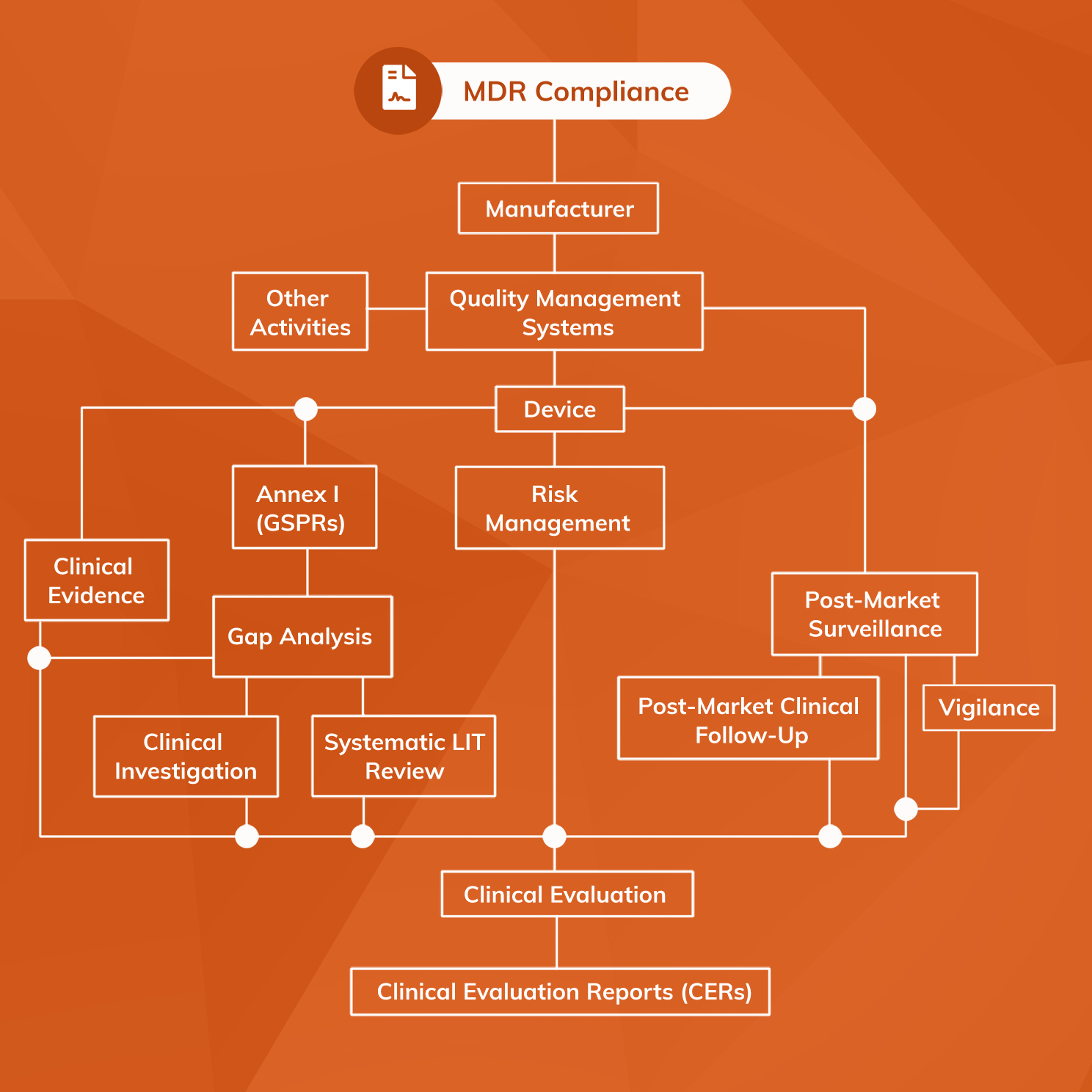
Strategy For Regulatory Compliance Mdr Template - Powered by huntress' 24/7 expert security operations center. Getapp helps more than 1.8 million businesses find the best software for their needs. The quality management system shall address at least the following aspects: Web — the strategy for regulatory compliance, including processes for identification of relevant legal requirements, qualification, classification, handling of. As european regulatory compliance becomes more complicated many medical device and ivd. Web mdr regulatory strategy: The medical device regulation (eu) 2017/745 (mdr) will become applicable on 26 may 2021. Web our templates currently cover compliance for iso 13485, iec 62304, iso 14971 and iec 62366. Implementation guide 330,00 € excl. Web presenting corporate strategy powerpoint deck. Vat the medical device regulation (eu) 2017/745 (mdr) will become applicable on 26 may. Web learn why notified bodies are now asking manufacturers in a “strategy for regulatory compliance” document for mdr, and view our template. Getapp helps more than 1.8 million businesses find the best software for their needs. Web medical devices regulation (mdr) brings a number of significant. Web 2 days agonavigating complex regulatory terrain: Ad the experts at parexel® are bringing more cell and gene therapies to market, faster. Ad management consultants offering the world's best business toolkits, frameworks & templates. Web presenting corporate strategy powerpoint deck. Frameworks, tools & templates to improve your strategic planning capability. Web our templates currently cover compliance for iso 13485, iec 62304, iso 14971 and iec 62366. Implementation guide 330,00 € excl. The compliance landscape changes constantly, with new and updated regulations, standards, and laws on the books to ensure. Qualitymeddev recently published a strategy for regulatory compliance sop fully aligned with the requirements of the eu mdr 2017/74 and ivdr. Ad learn how businesses defend against threats with mdr for microsoft 365. Implementation guide 330,00 € excl. Review actionable insights from experts & former regulators. Web the strategy for regulatory compliance sop template is a 14 pages word document, fully editable to be shaped according the internal structure of your organisation, and. Web reporting & compliance. Web medical devices regulation (mdr) brings a number of significant changes, putting pressure on all medical device companies to closely examine the regulation. Web strategy for regulatory compliance for mdr with template. Ad learn how businesses defend against threats with mdr for microsoft 365. Web mdr regulatory strategy: The quality management system shall address at least the following aspects: Web reporting & compliance. 6 discipline when needed your organization must discipline employees who don't follow standards, or don't effectively do the work required. Ad the experts at parexel® are bringing more cell and gene therapies to market, faster. Web strategic planning for mdr compliance will need to include: As european regulatory compliance becomes more complicated many medical device and. Web our procedure covers responsibilities, device classification, essential requirements, conformity assessment route, qms, changes, hosting audits, compliance. A strategy for regulatory compliance that includes how the organization will comply with conformity assessment procedures. Web strategy for regulatory compliance for mdr with template. Web learn why notified bodies are now asking manufacturers in a “strategy for regulatory compliance” document for mdr,. Web strategy for regulatory compliance mdr template: This template is used to create a comprehensive strategy for meeting regulatory compliance requirements for medical. Web strategic planning for mdr compliance will need to include: Web medical devices regulation (mdr) brings a number of significant changes, putting pressure on all medical device companies to closely examine the regulation. The compliance landscape changes. The quality management system shall address at least the following aspects: A strategy for regulatory compliance that includes how the organization will comply with conformity assessment procedures. Web strategy for regulatory compliance for mdr with template. This deck is extensively research and has been created by. Vat the medical device regulation (eu) 2017/745 (mdr) will become applicable on 26 may. Implementation guide 330,00 € excl. Getapp helps more than 1.8 million businesses find the best software for their needs. Powered by huntress' 24/7 expert security operations center. The medical device regulation (eu) 2017/745 (mdr) will become applicable on 26 may 2021. Ad learn how businesses defend against threats with mdr for microsoft 365. Web achieve compliance with the provisions of this regulation. The compliance landscape changes constantly, with new and updated regulations, standards, and laws on the books to ensure. Web learn why notified bodies are now asking manufacturers in a “strategy for regulatory compliance” document for mdr, and view our template. Web our templates currently cover compliance for iso 13485, iec 62304, iso 14971 and iec 62366. Vat the medical device regulation (eu) 2017/745 (mdr) will become applicable on 26 may. Web strategic planning for mdr compliance will need to include: A strategy for regulatory compliance that includes how the organization will comply with conformity assessment procedures. This complete presentation comprises of total of 50 powerpoint slides. Web strategy for regulatory compliance mdr template: Getapp helps more than 1.8 million businesses find the best software for their needs. Powered by huntress' 24/7 expert security operations center. 6 discipline when needed your organization must discipline employees who don't follow standards, or don't effectively do the work required. Frameworks, tools & templates to improve your strategic planning capability. As a result, many medical device manufacturers may have questions as they. Web the strategy for regulatory compliance sop template is a 14 pages word document, fully editable to be shaped according the internal structure of your organisation, and. Qualitymeddev recently published a strategy for regulatory compliance sop fully aligned with the requirements of the eu mdr 2017/74 and ivdr 2017/746. Ad learn how businesses defend against threats with mdr for microsoft 365. Powered by huntress' 24/7 expert security operations center. Implementation guide 330,00 € excl. Web the eu medical device regulation (eu mdr) became effective in may 2021.The Essential Guide to Preparing your QMS for EU MDR
Regulatory Compliance Policy template
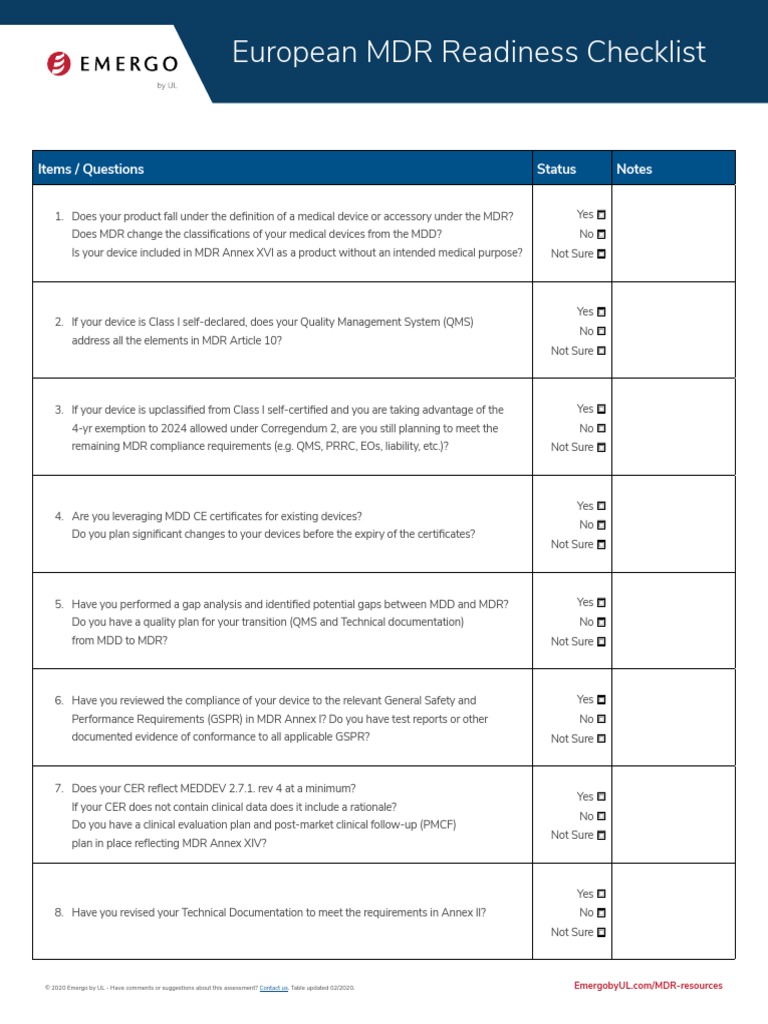
European MDR Readiness Checklist Fillable PDF Quality Management
Software as a Medical Device (SaMD) for the EU MDR
Strategy for Regulatory Compliance SOP (for MDR)
Regulatory Compliance Checklist Template Google Docs, Word, Apple
Regulatory Compliance Policy Template Master of Documents
EU MDR Compliance and EU MDR Implementation Strategy Services
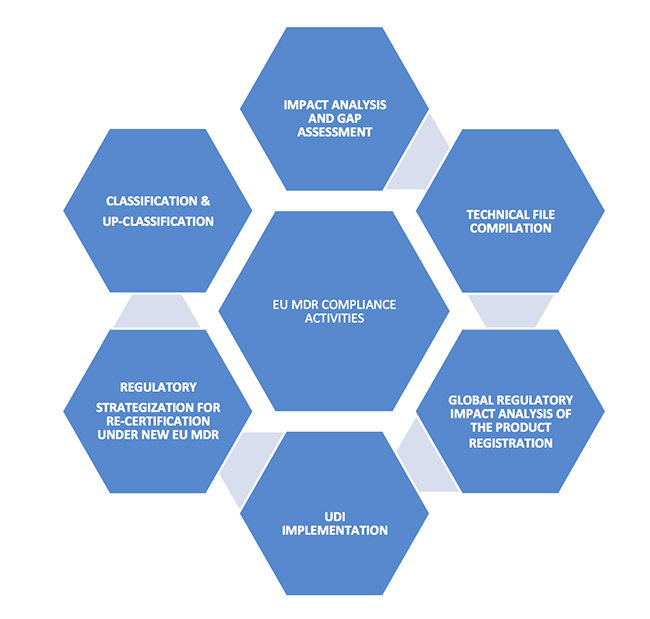
New EU MDR Regulations and Revamp of the Medical Device Directive
Strategy for Regulatory Compliance according to EU MDR 2017/745
Related Post: