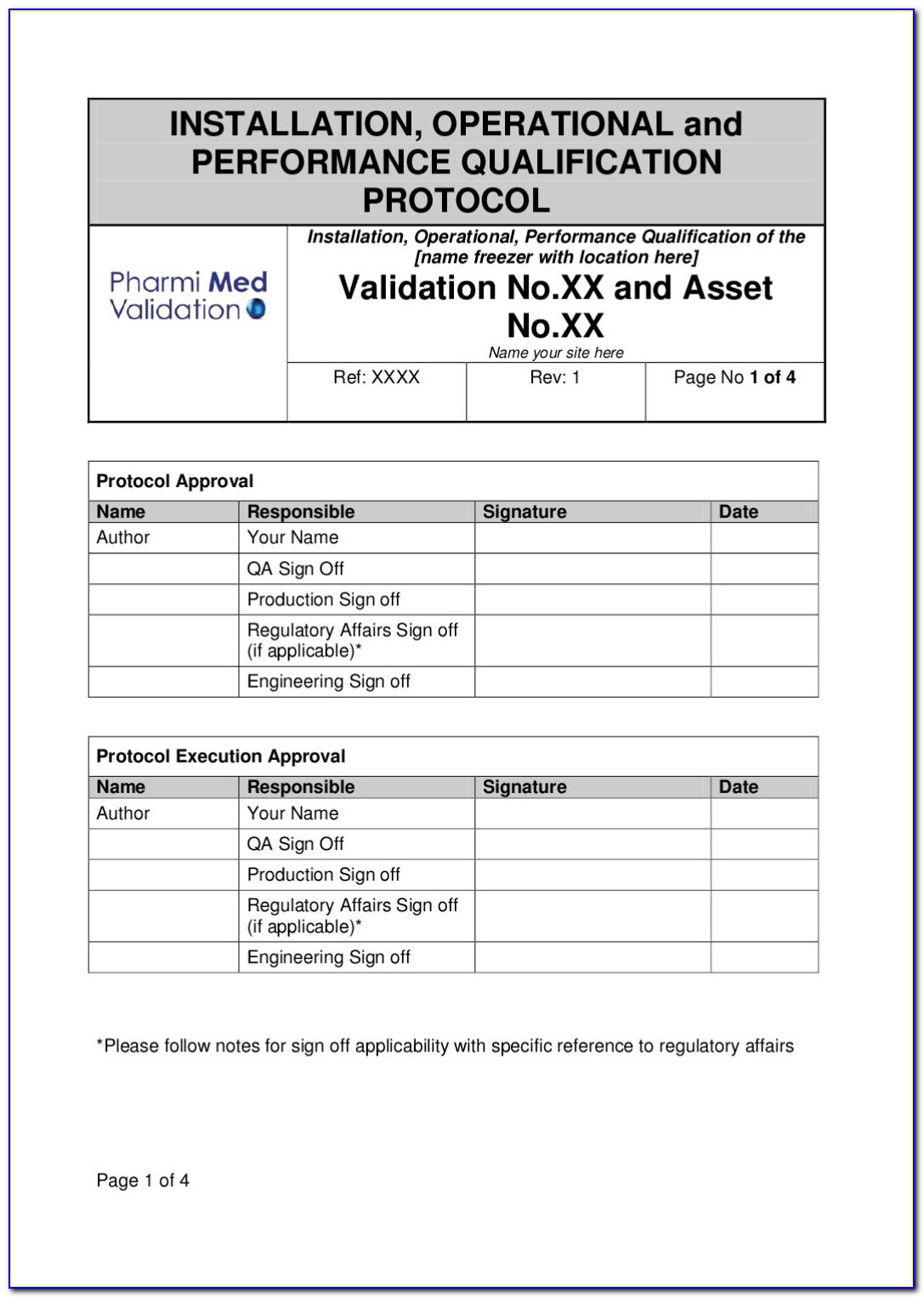
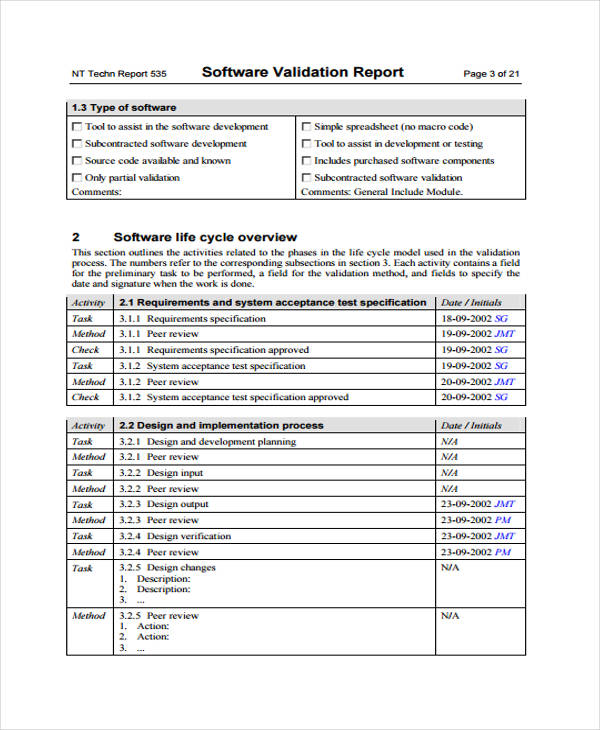
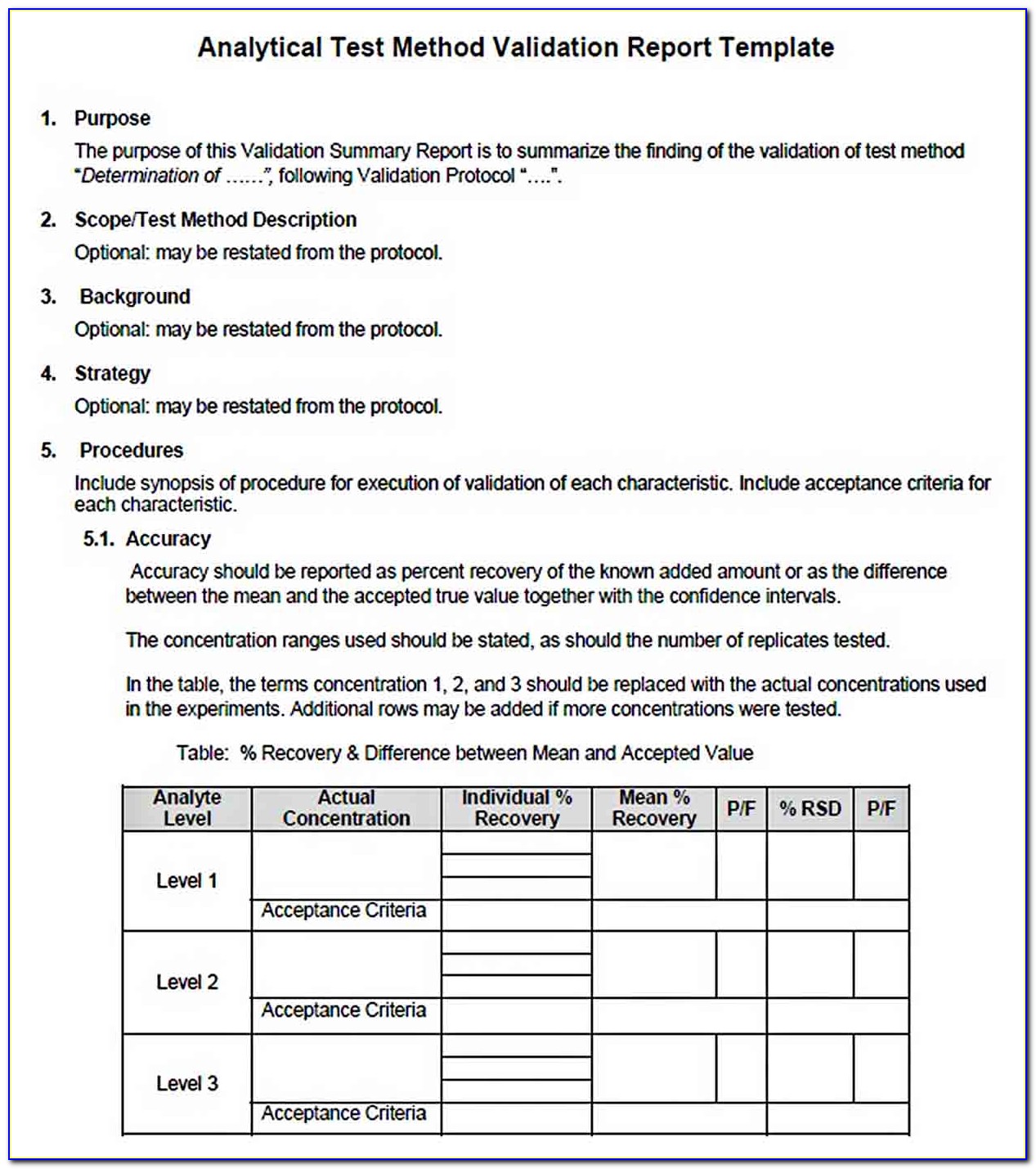
Software Validation Protocol Template
Software Validation Protocol Template - Basically, software verification activities consist of: It details factors such as product characteristics, production equipment,. It is imperative that all tests and inspections detailed in the validation template for vmp and. Drapes of scope are and validation project furthermore the strategy. Web page 2 guidance for industry and fda staff general principles of software validation in that case, the party with regulatory responsibility (i.e., the device manufacturer) needs to. The validation study documentation has been reviewed and approved. Ad digitize and manage any validation, commissioning or qualification process. Web software validation template and testing. On completion of each validation batch, a qualification report will be prepared. Web clickup's validation protocol sop template is designed to help you create and manage validation protocols for your standard operating procedures (sops). Validation of computer software is specified in section 4.1.6 of iso 13485:2016. The validation study documentation has been reviewed and approved. Trusted by leading pharma, biotech, and medical device companies globally. On completion of each validation batch, a qualification report will be prepared. View our free template press checklist. View our free template press checklist. Web same approval signatories as in the validation protocol & validation report. Web dort is a sample fda software validation template: Contains nonbinding recommendations (version october 6, 2021) 2 Web validation templates documentation interface. Web fda software validation is adenine complex procedures. Web an equipment validation protocol is a written plan stating how equipment qualification will be conducted. Contains nonbinding recommendations (version october 6, 2021) 2 Validate software which is used in. Web could be safety standard, regulatory standard, customer standards, or company standards. Trusted by leading pharma, biotech, and medical device companies globally. It is imperative that all tests and inspections detailed in the validation template for vmp and. Use this verification and validation plan template to review, inspect, test, audit, and establish whether items, processes, services. Web clickup's validation protocol sop template is designed to help you create and manage validation protocols. Web in house written software (excel/access) for eqr, sop and instrument data capture where validation is based on the risk and nature of the software. Basically, software verification activities consist of: Ad digitize and manage any validation, commissioning or qualification process. Validate software which is used in. Web software validation template and testing. It is imperative that all tests and inspections detailed in the validation template for vmp and. Web an equipment validation protocol is a written plan stating how equipment qualification will be conducted. The main messages there are: Use this verification and validation plan template to review, inspect, test, audit, and establish whether items, processes, services. It details factors such as. The validation study documentation has been reviewed and approved. It is imperative that all tests and inspections detailed in the validation template for vmp and. Use this verification and validation plan template to review, inspect, test, audit, and establish whether items, processes, services. Web clickup's validation protocol sop template is designed to help you create and manage validation protocols for. Drapes of scope are and validation project furthermore the strategy. Web same approval signatories as in the validation protocol & validation report. Web page 2 guidance for industry and fda staff general principles of software validation in that case, the party with regulatory responsibility (i.e., the device manufacturer) needs to. It details factors such as product characteristics, production equipment,. In. Web software validation template and testing. The main messages there are: Ad digitize and manage any validation, commissioning or qualification process. Methods validation is performed as per current industry guidelines cited in this sop. Web in house written software (excel/access) for eqr, sop and instrument data capture where validation is based on the risk and nature of the software. In this 2022 guide were tell what it is or how to verify software. Web software validation template and testing. An example validation package for an excel spreadsheet, including a functional specification, design. Web could be safety standard, regulatory standard, customer standards, or company standards. Basically, software verification activities consist of: The validation study documentation has been reviewed and approved. Web in house written software (excel/access) for eqr, sop and instrument data capture where validation is based on the risk and nature of the software. Drapes of scope are and validation project furthermore the strategy. On completion of each validation batch, a qualification report will be prepared. Web page 2 guidance for industry and fda staff general principles of software validation in that case, the party with regulatory responsibility (i.e., the device manufacturer) needs to. Validation of computer software is specified in section 4.1.6 of iso 13485:2016. It details factors such as product characteristics, production equipment,. Web software validation template and testing. Contains nonbinding recommendations (version october 6, 2021) 2 Trusted by leading pharma, biotech, and medical device companies globally. Web same approval signatories as in the validation protocol & validation report. Dynamic testing verifies the execution flow of software, including decision paths, inputs, and. The main messages there are: Methods validation is performed as per current industry guidelines cited in this sop. Use this verification and validation plan template to review, inspect, test, audit, and establish whether items, processes, services. Basically, software verification activities consist of: Web fda software validation is adenine complex procedures. In this 2022 guide were tell what it is or how to verify software. An example validation package for an excel spreadsheet, including a functional specification, design. Web validation templates documentation interface.10+ Validation Report Templates Free Sample, Example Format Download
Software Validation Procedure Template
10+ Validation Plan Templates Sample Templates
Template Word Master Software Validation Test Plan according to the
Software Validation Test Plan Template [Free PDF] Google Docs, Word
Software Validation Templates
Analytical Test Method Validation Protocol Template
Iq Oq Pq Software Validation Templates
10+ Validation Report Templates Free Sample, Example Format Download
Analytical Method Validation Protocol Sample
Related Post:
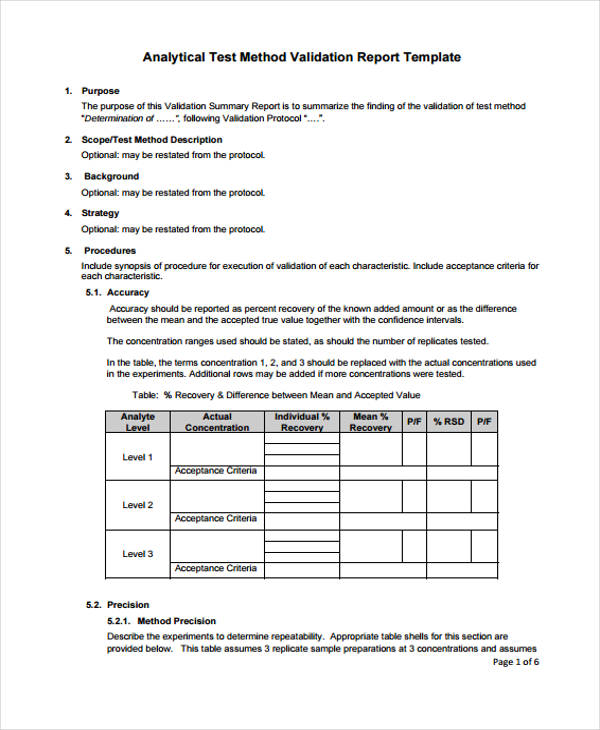



![Software Validation Test Plan Template [Free PDF] Google Docs, Word](https://images.template.net/63676/Software-Validation-Test-Plan-Template-1.jpeg)