Sfdr Periodic Reporting Template
Sfdr Periodic Reporting Template - Web following the publication in the eu official journal of regulation of commission delegated regulation (eu) 2022/1288 supplementing the sustainable. The main provisions of the disclosure regulation will apply from 10 march 2021. Aside from this outline, there are downloadable safety report templates that you can fill in to make a suitable safety report for a given time and type. Use a doc example for a heart patient, hosptial doctor, personal. Web a document called a safety report evaluates or informs the risks and security of a phenomenon, procedure, service, and product. The role of the patient safety. In the draft rts the esas. Web on 6 april 2022, the european commission adopted the final regulatory technical standards (rts) under the sustainable finance disclosure regulation. In other words, it is a report about a. Web amended disclosure templates. Article 11(2) of the regulation. When will the disclosure regulation apply? Web 7+ medical summary report templates. Web product disclosure in periodic reports: Web sfdr imposes significant esg disclosure obligations on asset managers marketing funds in the eu. Web as authorized by the secretary of hhs, ahrq coordinates the development of common definitions and reporting formats (common formats) for reporting on health. In the draft rts the esas. Web sfdr imposes significant esg disclosure obligations on asset managers marketing funds in the eu. Web the european supervisory authorities (esas) have developed through the joint committee (jc) draft regulatory. Article 11(2) of the regulation. Web a document called a safety report evaluates or informs the risks and security of a phenomenon, procedure, service, and product. Web following the publication in the eu official journal of regulation of commission delegated regulation (eu) 2022/1288 supplementing the sustainable. In the draft rts the esas. Web as authorized by the secretary of hhs,. Web product disclosure in periodic reports: Web as authorized by the secretary of hhs, ahrq coordinates the development of common definitions and reporting formats (common formats) for reporting on health. In the draft rts the esas. Article 11(2) of the regulation. Web 7+ medical summary report templates. Web following the publication in the eu official journal of regulation of commission delegated regulation (eu) 2022/1288 supplementing the sustainable. Web sfdr imposes significant esg disclosure obligations on asset managers marketing funds in the eu. They set out detailed disclosure requirements under sfdr and the taxonomy regulation and provide. Web as authorized by the secretary of hhs, ahrq coordinates the. They set out detailed disclosure requirements under sfdr and the taxonomy regulation and provide. Use a doc example for a heart patient, hosptial doctor, personal. In other words, it is a report about a. The role of the patient safety. Web on 2 june 2022, following what they describe as “numerous requests for clarifications received from stakeholders and national competent. Article 11(2) of the regulation. Web on 6 april 2022, the european commission adopted the final regulatory technical standards (rts) under the sustainable finance disclosure regulation. Web the european supervisory authorities (esas) have developed through the joint committee (jc) draft regulatory technical standards (rts) with regard to the content and. Web as authorized by the secretary of hhs, ahrq coordinates. Web amended disclosure templates. Web following the publication in the eu official journal of regulation of commission delegated regulation (eu) 2022/1288 supplementing the sustainable. When will the disclosure regulation apply? Web 7+ medical summary report templates. The main provisions of the disclosure regulation will apply from 10 march 2021. Templates are provided in annexes iv and v in the final report of the esas and the draft rts. Web sfdr imposes significant esg disclosure obligations on asset managers marketing funds in the eu. Web disclosures outlined in sfdr and the taxonomy regulation. In other words, it is a report about a. Article 11(2) of the regulation. Web disclosures outlined in sfdr and the taxonomy regulation. The main provisions of the disclosure regulation will apply from 10 march 2021. Web on 2 june 2022, following what they describe as “numerous requests for clarifications received from stakeholders and national competent authorities”, the european. Grab a free download sample when preparing a medical report. Templates are provided in annexes. They set out detailed disclosure requirements under sfdr and the taxonomy regulation and provide. Web disclosures outlined in sfdr and the taxonomy regulation. Article 11(2) of the regulation. Web product disclosure in periodic reports: In the draft rts the esas. Web a document called a safety report evaluates or informs the risks and security of a phenomenon, procedure, service, and product. Web on 6 april 2022, the european commission adopted the final regulatory technical standards (rts) under the sustainable finance disclosure regulation. Use a doc example for a heart patient, hosptial doctor, personal. Web as authorized by the secretary of hhs, ahrq coordinates the development of common definitions and reporting formats (common formats) for reporting on health. Web sfdr imposes significant esg disclosure obligations on asset managers marketing funds in the eu. Templates are provided in annexes iv and v in the final report of the esas and the draft rts. Aside from this outline, there are downloadable safety report templates that you can fill in to make a suitable safety report for a given time and type. Web following the publication in the eu official journal of regulation of commission delegated regulation (eu) 2022/1288 supplementing the sustainable. The role of the patient safety. Web on 2 june 2022, following what they describe as “numerous requests for clarifications received from stakeholders and national competent authorities”, the european. Web amended disclosure templates. In other words, it is a report about a. Grab a free download sample when preparing a medical report. Web 7+ medical summary report templates. When will the disclosure regulation apply?REG.8.20105 Periodic_Safety_Update_Report
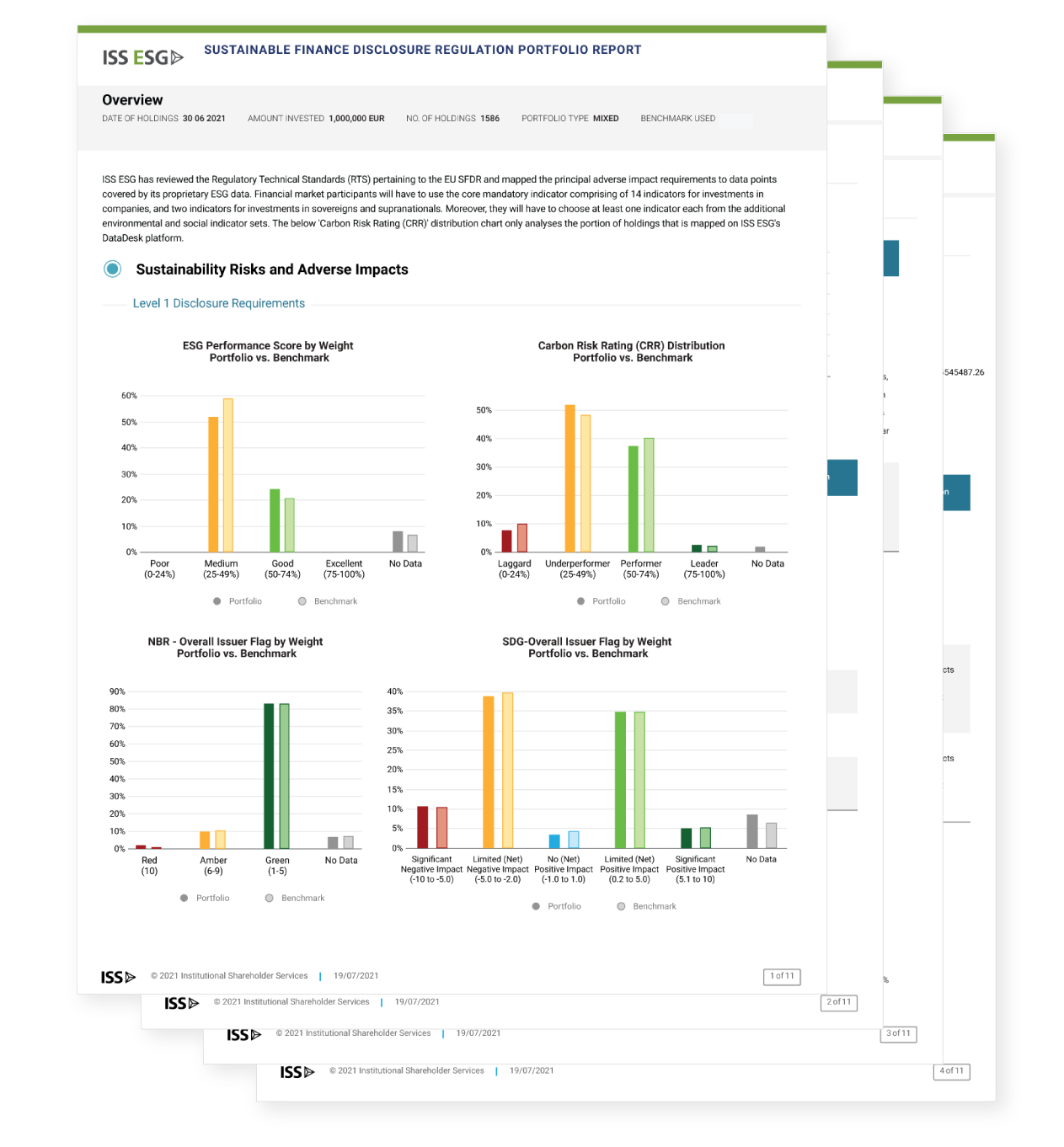
SFDR Principal Adverse Impact Solution ISS
SFDR Principal Adverse Impact
Fillable Transmittal Of Periodic Reports printable pdf download
SFDR EY Luxembourg
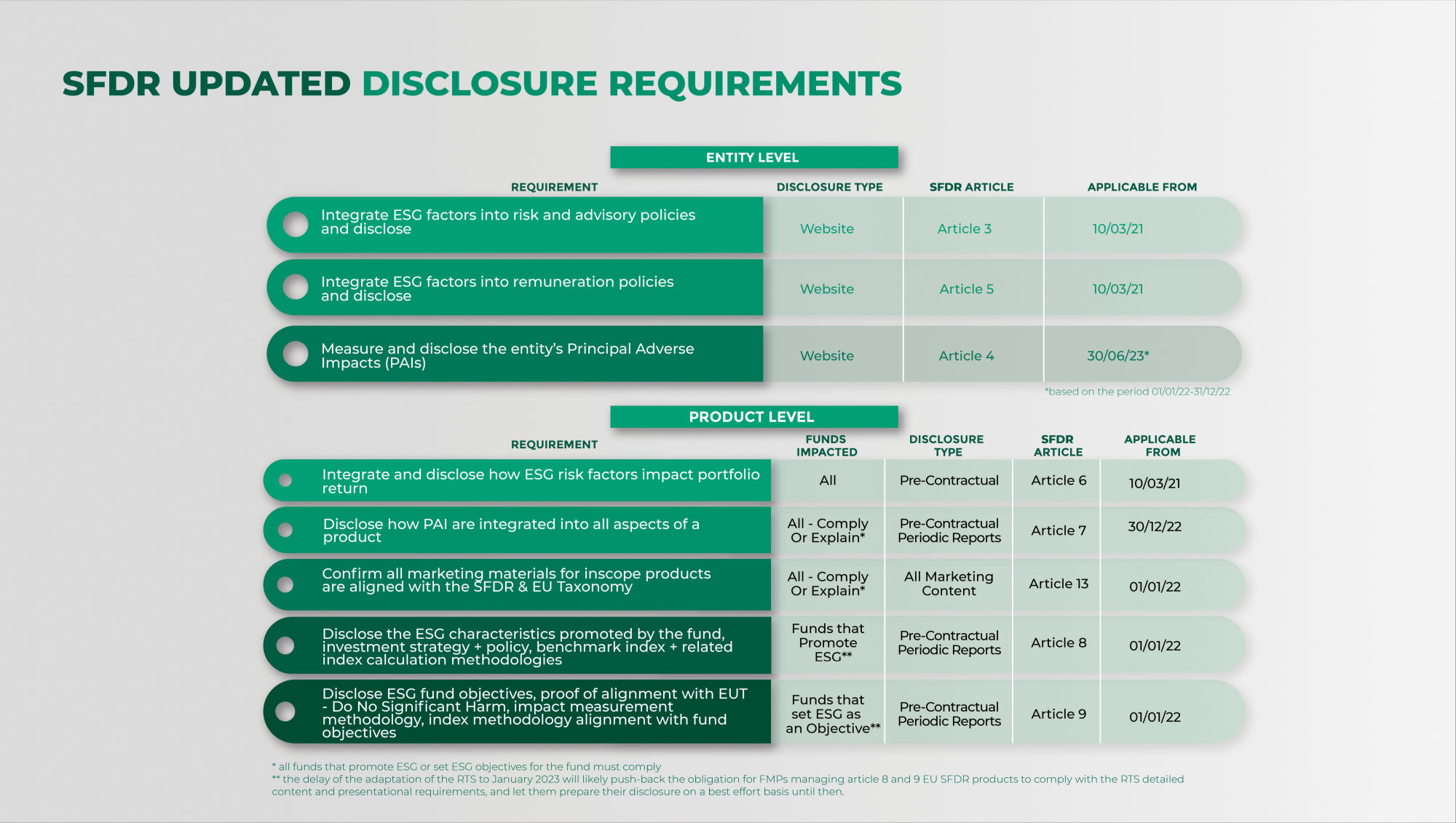
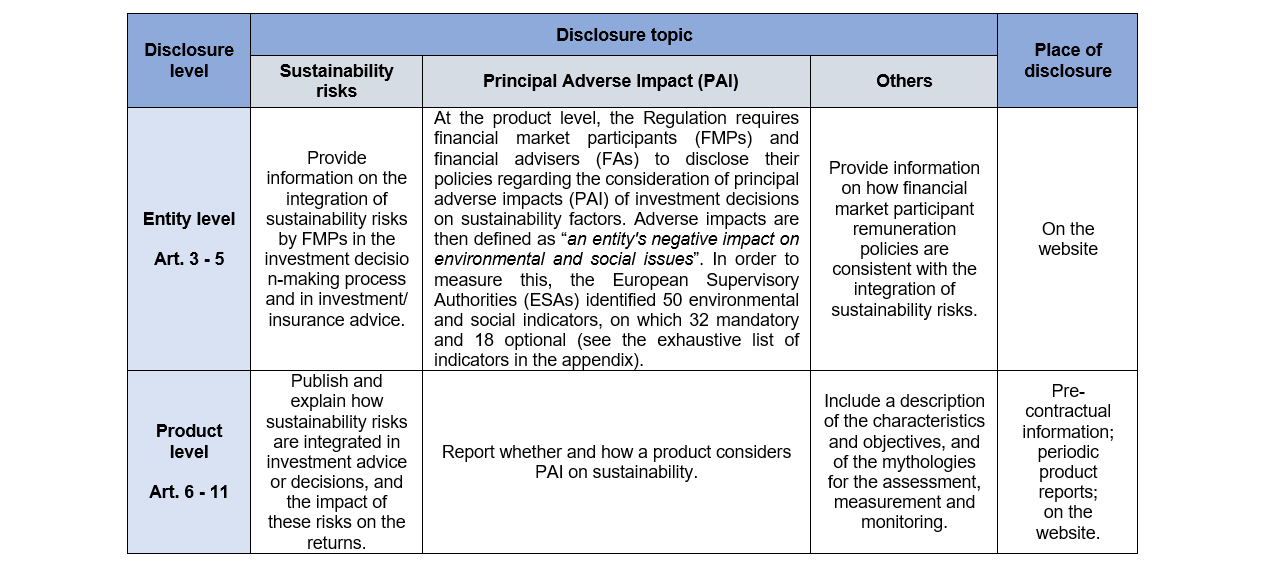
The sustainable finance disclosure regulation (SFDR) enhancing clarity
Fda Periodic Safety Update Reports
SFDR Fundamentals, Challenges and Keys to Success
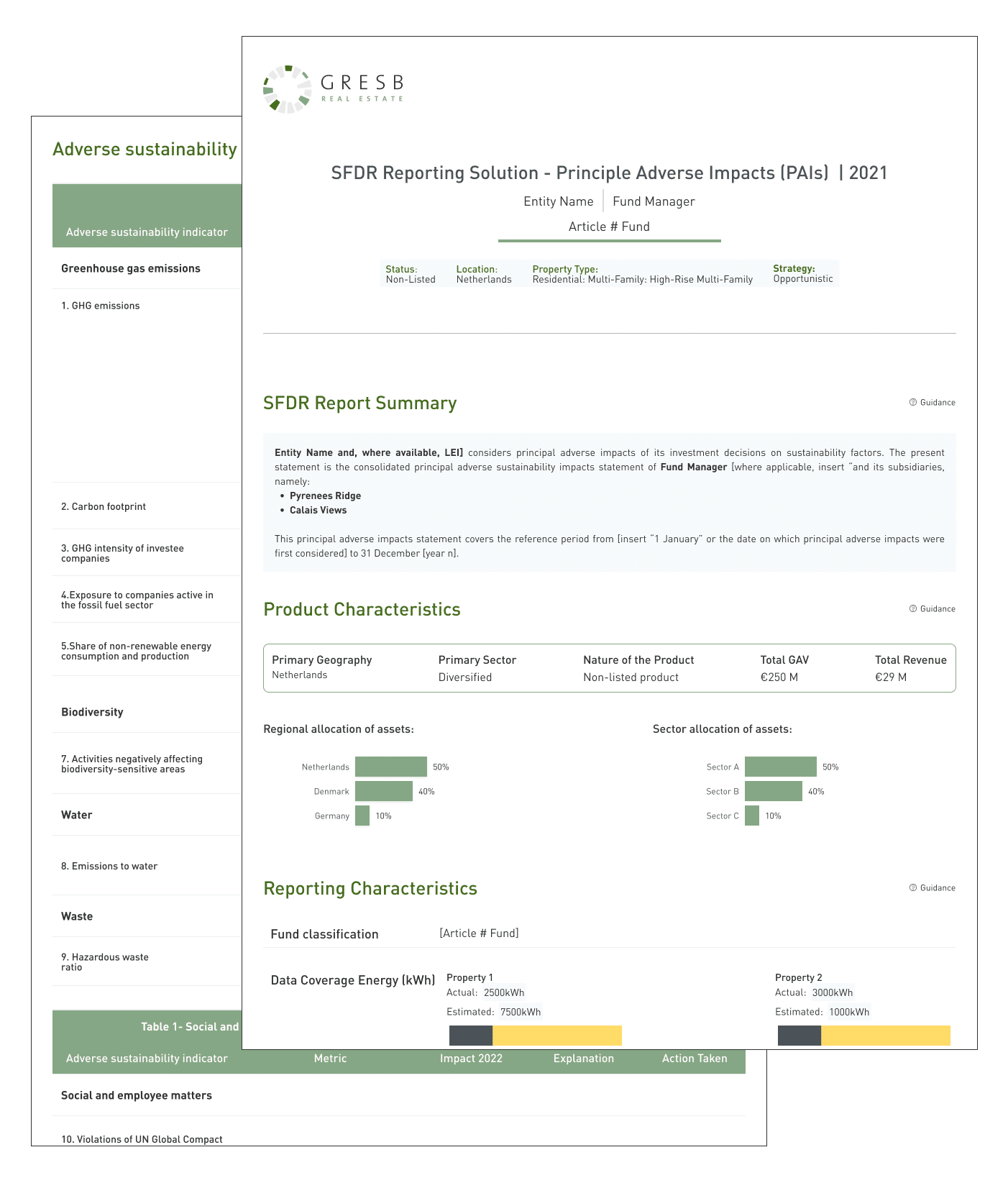
SFDR Reporting Solution GRESB
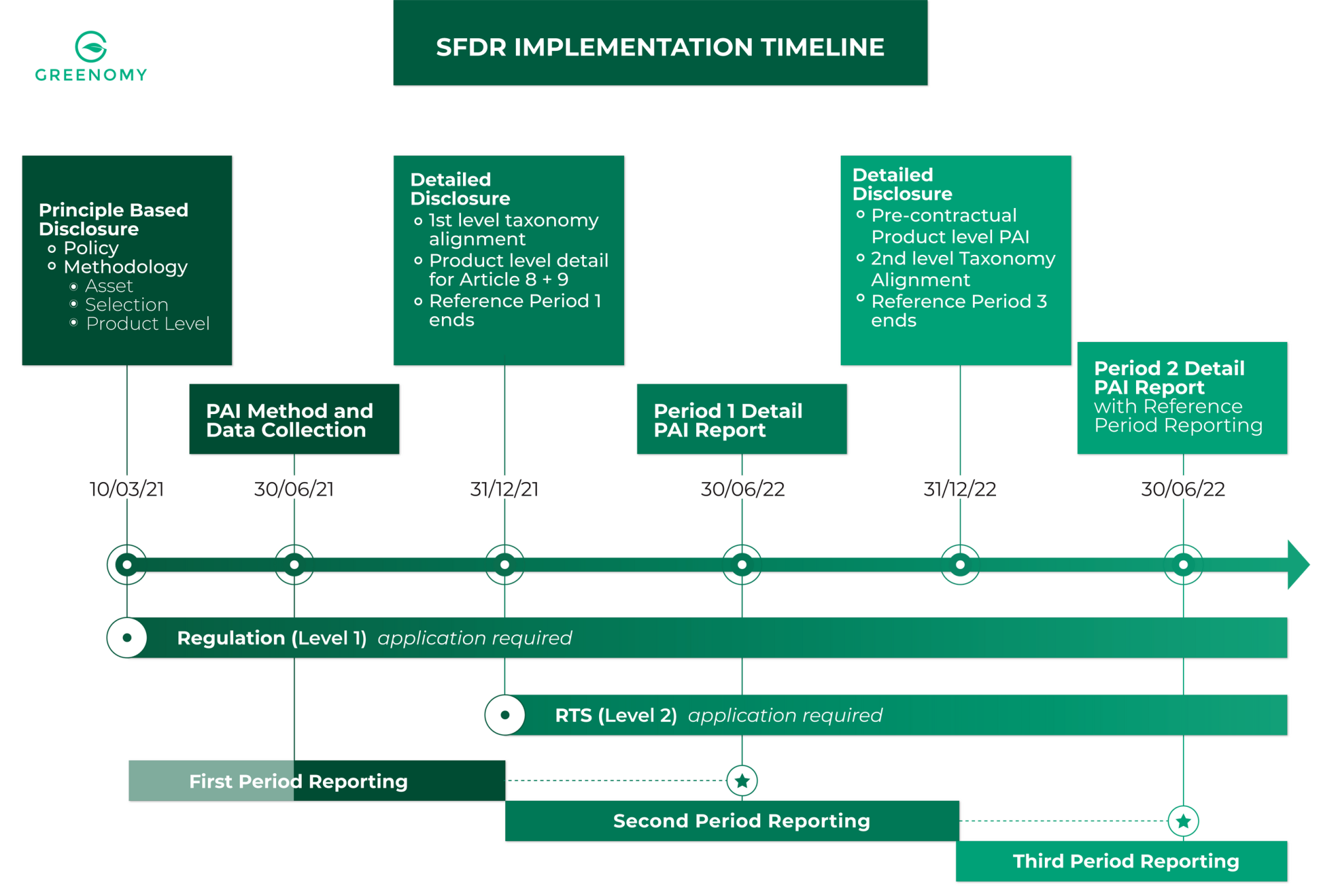
SFDR reporting timeline what you have to disclose and when
Related Post: