Psur Template
Psur Template - This form details information regarding the medical device,. The required format and content of psurs in the eu are based on those for the periodic benefit risk. Below we have provided an overview of the document; Template that contains all the information that will be available in eudamed for the psur. Ad edit, fill & esign pdf documents online. Web in november 1996, the ich endorsed the ich e2c periodic safety update report guideline (ich e2c(r1) guideline), which established the psur as a harmonized format for. Web this guideline on the format and content of periodic safety update reports (psurs) is considered particulary suitable for comprehensive reports covering short periods (e.g. The p eriodic safety update report (psur template) is one of the new requirements related to the european medical device regulation eu mdr 2017/745. In annex ii, tables are provided on. Web the scope, objectives, format and content of the psur are described in vii.b. Below we have provided an overview of the document; Web periodic safety update report (psur) was on relevant new safety information in the context of patient exposure, to determine if changes were needed to the reference. This form details information regarding the medical device,. We have developed high quality psur template, procedures and forms for periodic safety update reports in. Web the scope, objectives, format and content of the psur are described in vii.b. What is the purpose of a. However, the full guidance should be consulted when drafting your psur. Web this guideline on the format and content of periodic safety update reports (psurs) is considered particulary suitable for comprehensive reports covering short periods (e.g. Below we have provided. Best pdf fillable form builder. What is the purpose of a. We have developed high quality psur template, procedures and forms for periodic safety update reports in compliance with mdr. Web cover playstation 5 / deco for ps5 cross, round, square, triangle with this 3d model, add a touch of design on your playstation 5. Web this guideline on the. The p eriodic safety update report (psur template) is one of the new requirements related to the european medical device regulation eu mdr 2017/745. We have developed high quality psur template, procedures and forms for periodic safety update reports in compliance with mdr. Below we have provided an overview of the document; Web the scope, objectives, format and content of. Web periodic safety update report (psur) was on relevant new safety information in the context of patient exposure, to determine if changes were needed to the reference. Below we have provided an overview of the document; In annex ii, tables are provided on. Web in november 1996, the ich endorsed the ich e2c periodic safety update report guideline (ich e2c(r1). Web psur template, procedure and sop. Web this guideline on the format and content of periodic safety update reports (psurs) is considered particulary suitable for comprehensive reports covering short periods (e.g. Below we have provided an overview of the document; Ad edit, fill & esign pdf documents online. The p eriodic safety update report (psur template) is one of the. However, the full guidance should be consulted when drafting your psur. Web periodic safety update report (psur) was on relevant new safety information in the context of patient exposure, to determine if changes were needed to the reference. Web cover playstation 5 / deco for ps5 cross, round, square, triangle with this 3d model, add a touch of design on. However, the full guidance should be consulted when drafting your psur. Web periodic safety update report (psur) was on relevant new safety information in the context of patient exposure, to determine if changes were needed to the reference. Best pdf fillable form builder. This form details information regarding the medical device,. Ad edit, fill & esign pdf documents online. However, the full guidance should be consulted when drafting your psur. Template that contains all the information that will be available in eudamed for the psur. Web in november 1996, the ich endorsed the ich e2c periodic safety update report guideline (ich e2c(r1) guideline), which established the psur as a harmonized format for. Web a free resource to help you. The p eriodic safety update report (psur template) is one of the new requirements related to the european medical device regulation eu mdr 2017/745. Web a free resource to help you document all necessary components that must be included in your periodic safety update report (psur) in accordance with the requirements of eu. Web periodic safety update report (psur) was. The required format and content of psurs in the eu are based on those for the periodic benefit risk. Web psur template, procedure and sop. In annex ii, tables are provided on. Template that contains all the information that will be available in eudamed for the psur. Web this guideline on the format and content of periodic safety update reports (psurs) is considered particulary suitable for comprehensive reports covering short periods (e.g. Web this guidance describes the conditions under which applicants can use an alternative reporting format, the international council for harmonisation (ich)3 e2c (r2). What is the purpose of a. The p eriodic safety update report (psur template) is one of the new requirements related to the european medical device regulation eu mdr 2017/745. This form details information regarding the medical device,. Ad edit, fill & esign pdf documents online. Web the scope, objectives, format and content of the psur are described in vii.b. Web in november 1996, the ich endorsed the ich e2c periodic safety update report guideline (ich e2c(r1) guideline), which established the psur as a harmonized format for. Below we have provided an overview of the document; However, the full guidance should be consulted when drafting your psur. Best pdf fillable form builder. Web a free resource to help you document all necessary components that must be included in your periodic safety update report (psur) in accordance with the requirements of eu. Web cover playstation 5 / deco for ps5 cross, round, square, triangle with this 3d model, add a touch of design on your playstation 5. We have developed high quality psur template, procedures and forms for periodic safety update reports in compliance with mdr. Web periodic safety update report (psur) was on relevant new safety information in the context of patient exposure, to determine if changes were needed to the reference.PSUR Requirements
Creating a Periodic Safety Update Report (PSUR) that Complies with MDR
Periodic Safety Update Report (PSUR) Template QualityMedDev
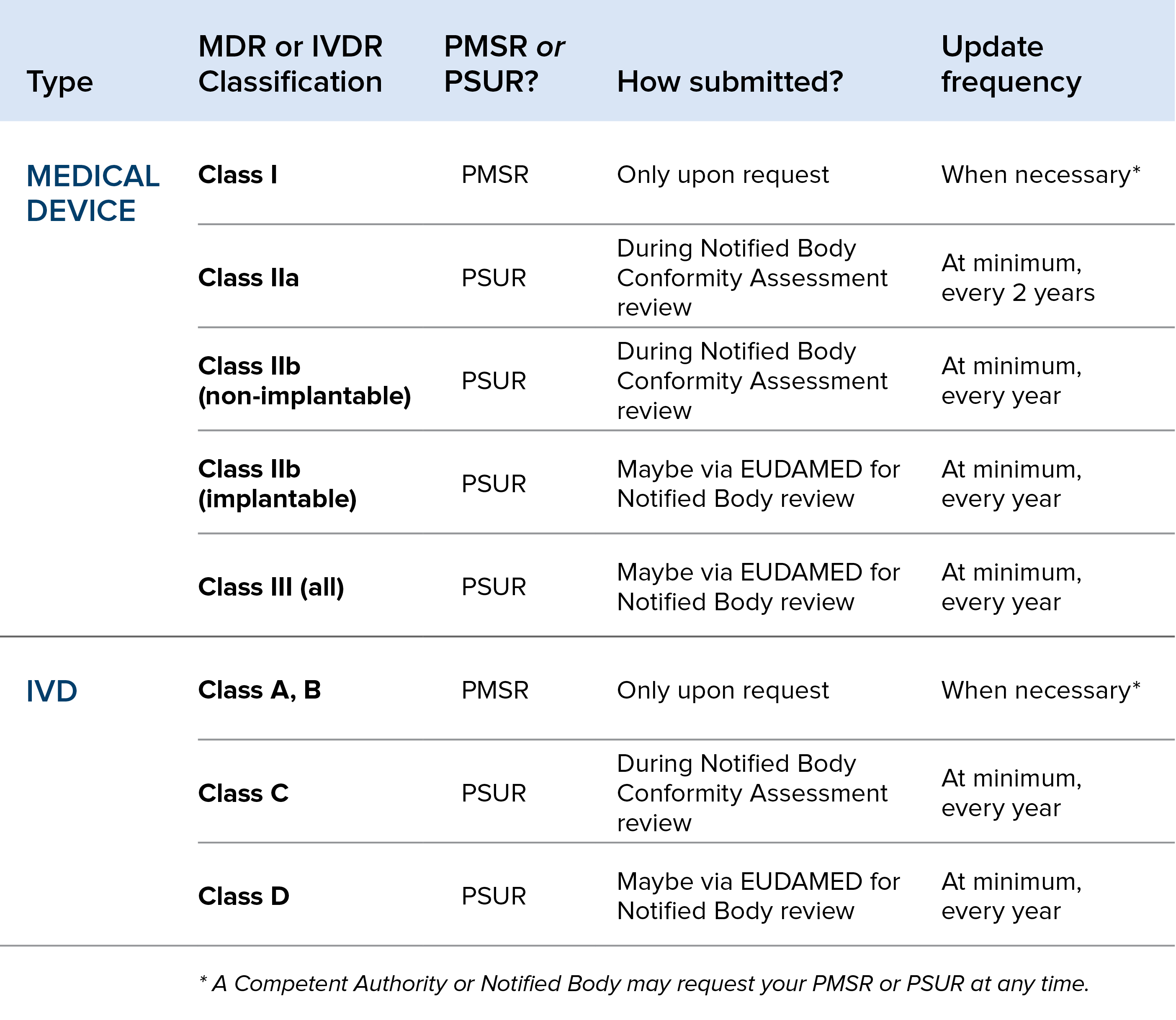
PSUR Requirements
PSUR Requirements
Periodic Safety Update Report (PSUR) Procedure
Psur Template PDF Pharmacovigilance Clinical Trial
Requirements For European MDR PSUR & PMSR Oriel STAT A MATRIX
PSUR Assessment Report template for use by the European
Periodic Survey Update Template by Pharmi Med Ltd Issuu
Related Post:

 that Complies with MDR & IVDR.png)