Protocol Template Word
Protocol Template Word - All the details of the main investigator must be reported in the first paragraph. Web marchi, anthony (nih/od) [c], last modified by rasmussen, kevin (nih/od) [c] jan 26,. Web these are locked in the downloadable template so they are not removed. Biomedical protocol template (word document) nih new clinical trial. Web generic informed consent template. Web the ich m11 clinical electronic structured harmonised protocol. Web the university of warwick's protocol template is available below and is a. Web instructions download the template (s) of your choice. Description of the core center, contacts of the investigator/s, quantification of the involved centers. The practice utilizes a written client conflict protocol to help effectively address upset. Web which protocol template should you use? Web generic informed consent template. Ad easy to use project management. Web clinical trial protocol cfty720dus40 / nct03257358. The practice utilizes a written client conflict protocol to help effectively address upset. Ad easy to use project management. Description of the core center, contacts of the investigator/s, quantification of the involved centers. Web listed below are several templates to assist you in composing your. Web these are locked in the downloadable template so they are not removed. Web resources for our new common protocol template is now accessible via download. Web ðï ࡱ á> þÿ = ; Web this page has checklists and templates to help you write your protocol. All the details of the main investigator must be reported in the first paragraph. Web listed below are several templates to assist you in composing your. Web the irb provides several protocol templates on this page. The template documents open as. Web open in a separate window. Web resources for our new common protocol template is now accessible via download. Web ðï ࡱ á> þÿ = ; Web phase 1 clinical trial protocol template. Web these are locked in the downloadable template so they are not removed. Biomedical protocol template (word document) nih new clinical trial. The practice utilizes a written client conflict protocol to help effectively address upset. Web which protocol template should you use? Web this page has checklists and templates to help you write your protocol. Web this link downloads as a microsoft word document detailing the specific. Using the pmi cdisc ec templates to build a protocol‐specific ec. Web these are locked in the downloadable template so they are not removed. The template documents open as. Web ðï ࡱ á> þÿ = ; All the details of the main investigator must be reported in the first paragraph. Web clinical trial protocol cfty720dus40 / nct03257358. Web these are locked in the downloadable template so they are not removed. The template documents open as. Web the irb provides several protocol templates on this page. Web generic informed consent template. The template documents open as. Web which protocol template should you use? The irb office has developed protocol. Web the university of warwick's protocol template is available below and is a. The irb office has developed protocol. Web marchi, anthony (nih/od) [c], last modified by rasmussen, kevin (nih/od) [c] jan 26,. Web these are locked in the downloadable template so they are not removed. Web ðï ࡱ á> þÿ r t. Download link (microsoft word document): Web ðï ࡱ á> þÿ r t. Web word versions of the protocol templates can also be downloaded for use. Web clinical trial protocol cfty720dus40 / nct03257358. Web open in a separate window. Web resources for our new common protocol template is now accessible via download. Web ðï ࡱ á> þÿ = ; Web the ich m11 clinical electronic structured harmonised protocol. Web this link downloads as a microsoft word document detailing the specific. Web open in a separate window. A research protocol must start from the definition of the coordinator of the whole study: Web the irb provides several protocol templates on this page. Download link (microsoft word document): Web these are locked in the downloadable template so they are not removed. Web word versions of the protocol templates can also be downloaded for use. Web marchi, anthony (nih/od) [c], last modified by rasmussen, kevin (nih/od) [c] jan 26,. Web instructions download the template (s) of your choice. Web generic informed consent template. The irb office has developed protocol. Web which protocol template should you use? The item identifiers are slightly out of sequence to make the document flow more easily but it is important that they remain in the document to allow electronic searches by spirit item number. The template documents open as. The practice utilizes a written client conflict protocol to help effectively address upset. All the details of the main investigator must be reported in the first paragraph. Description of the core center, contacts of the investigator/s, quantification of the involved centers. Using the pmi cdisc ec templates to build a protocol‐specific ec.Procedure Template MS Word Standard Operating Procedure & SOP forms
Template Device protocol
Procedure Manual Template Word Free Best Professional Templates
KHP CTU Protocol Template
Top 8 Protocol Templates free to download in PDF format
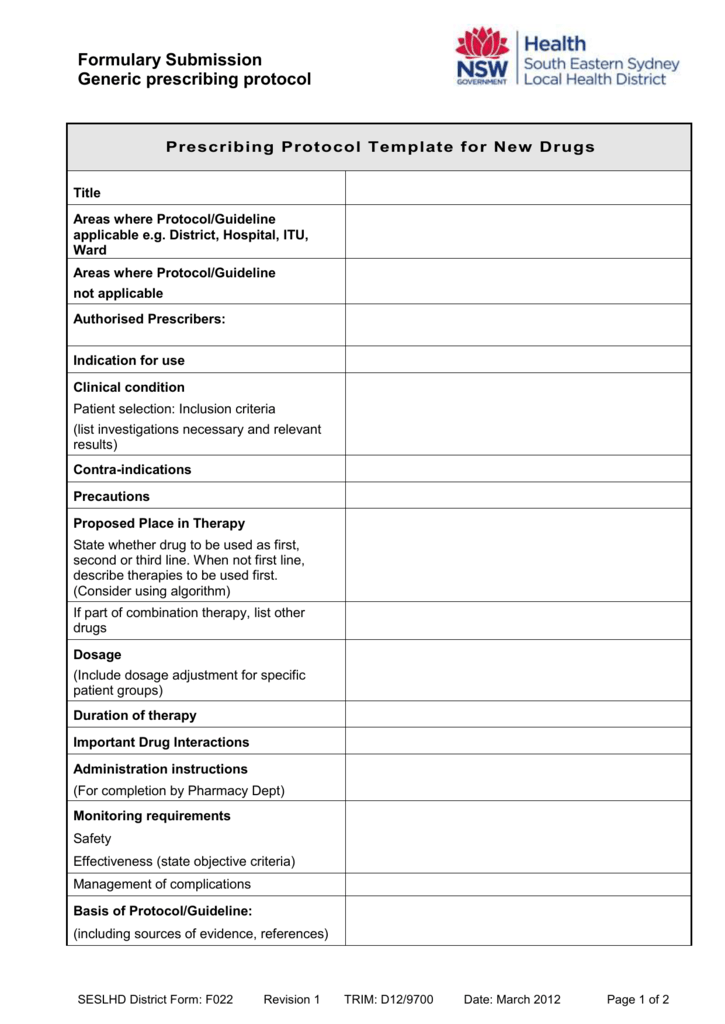
Prescribing Protocol Template for New Drugs
10 Make Free Standard Operating Procedure In Word SampleTemplatess
Procedure Template MS Word Standard Operating Procedure & SOP forms
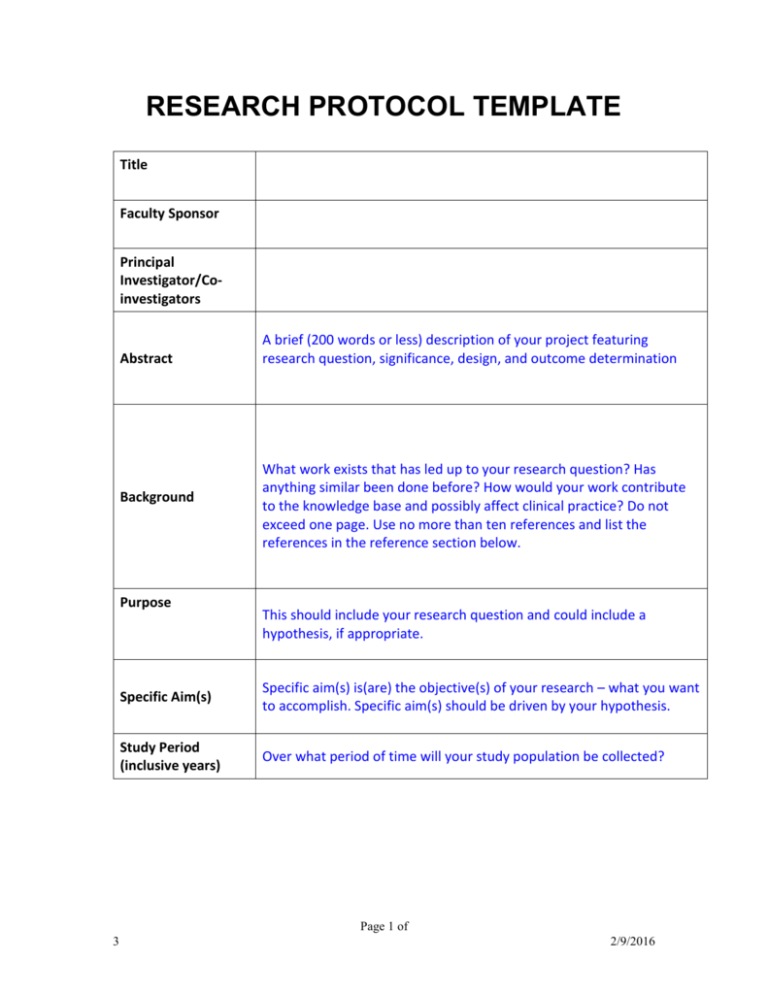
research protocol template
Quality Acceptance Protocol
Related Post: