Phase 1 Clinical Trial Protocol Template
Phase 1 Clinical Trial Protocol Template - For nonclinical research or clinical trials that are phase 0 or phase 1, use this free template. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a food. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human. Early developmental protocols should specify in detail all. Choose from thousands of templates like treatment plans, interventions, and more. 57 7.3.2 sample size in phase 2 and phase. Web this template aims to facilitate the development of phase 2 and 3 clinical trial protocols that require a food and drug administration (fda) investigational new drug (ind) or. Web suggested templates for phase 1 and 2 clinical trials. Clinical trial protocol version number: Center for drug evaluation and research, office of regulatory policy. Generic protocol documents and instructions for ctep studies. 7.3.1 sample size in phase 1 clinical trials. 00 (original protocol) development phase: Web this trial protocol has been provided by the authors to give readers additional information about their work. Ad put the experience of our mobile research nurses to work for your trial. Web suggested templates for phase 1 and 2 clinical trials response evaluation criteria in solid tumors (recist) — sites can voluntarily incorporate the. 0.1 template revision history date description of revision. 7.3.1 sample size in phase 1 clinical trials. This template is intended for interventional clinical trials of. Clinical trial protocol version number: Interventional clinical trial protocol template 2. 57 7.3.2 sample size in phase 2 and phase. Web phase 1 clinical trial protocol template. Web this trial protocol has been provided by the authors to give readers additional information about their work. The interventional drug/device trial template and the behavioral and social science research template. Choose from thousands of templates like treatment plans, interventions, and more. Web when submitting a new clinical protocol as part of an ind application, consider the following: 7.3.1 sample size in phase 1 clinical trials. This template is intended for interventional clinical trials of. 00 (original protocol) clinical trial phase: Web 3 rows this protocol template aims to facilitate the development of two types of clinical trials. Web this template aims to facilitate the development of phase 2 and 3 clinical trial protocols that require a food and drug administration (fda) investigational new drug (ind) or. Web there are two templates to be used for interventional research: Choose from thousands. Web this template aims to facilitate the development of phase 2 and 3 clinical trial protocols that require a food and drug administration (fda) investigational new drug (ind) or. 57 7.3.2 sample size in phase 2 and phase. The template is suitable for. 7.3.1 sample size in phase 1 clinical trials. Web phase 1 clinical trial protocol template. Web suggested templates for phase 1 and 2 clinical trials. Web suggested templates for phase 1 and 2 clinical trials response evaluation criteria in solid tumors (recist) — sites can voluntarily incorporate the. Mobile research nurses drive shift to decentralized trials. Choose from thousands of templates like treatment plans, interventions, and more. 57 7.3.2 sample size in phase 2 and. For nonclinical research or clinical trials that are phase 0 or phase 1, use this free template. Web this section of the clinical trial agreement template provides you with an area to document all ownership rights between parties as well as any other rights to property involved in. Web this clinical trial protocol template is a suggested format for phase. For nonclinical research or clinical trials that are phase 0 or phase 1, use this free template. Web when submitting a new clinical protocol as part of an ind application, consider the following: Early developmental protocols should specify in detail all. Web this section of the clinical trial agreement template provides you with an area to document all ownership rights. Web this trial protocol has been provided by the authors to give readers additional information about their work. 00 (original protocol) clinical trial phase: The sample size for part 1 of this study was determined by clinical rather than statistical considerations. Web 2.2 phase 1 clinical trial protocol. Web the clinical trials protocol template for the behavioral and social sciences. Center for drug evaluation and research, office of regulatory policy. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human. 00 (original protocol) clinical trial phase: 7.3.1 sample size in phase 1 clinical trials. Web this section of the clinical trial agreement template provides you with an area to document all ownership rights between parties as well as any other rights to property involved in. The interventional drug/device trial template and the behavioral and social science research template. Web 2.2 phase 1 clinical trial protocol. 0.1 template revision history date description of revision. Web this template aims to facilitate the development of phase 2 and 3 clinical trial protocols that require a food and drug administration (fda) investigational new drug (ind) or. This template is intended for interventional clinical trials of. Web the clinical trials protocol template for the behavioral and social sciences is a resource for communicating the science, methods, and operations of a clinical trial. The template is suitable for. Interventional clinical trial protocol template 2. Mobile research nurses drive shift to decentralized trials. The sample size for part 1 of this study was determined by clinical rather than statistical considerations. Web phase 1 clinical trial protocol template. Web suggested templates for phase 1 and 2 clinical trials. Web suggested templates for phase 1 and 2 clinical trials response evaluation criteria in solid tumors (recist) — sites can voluntarily incorporate the. Ad put the experience of our mobile research nurses to work for your trial. For nonclinical research or clinical trials that are phase 0 or phase 1, use this free template.Clinical Trial Protocol Template Eu Templates NjQyMjk Resume Examples
Process for determining clinical trial design of phase 1
Clinical Trial Protocol
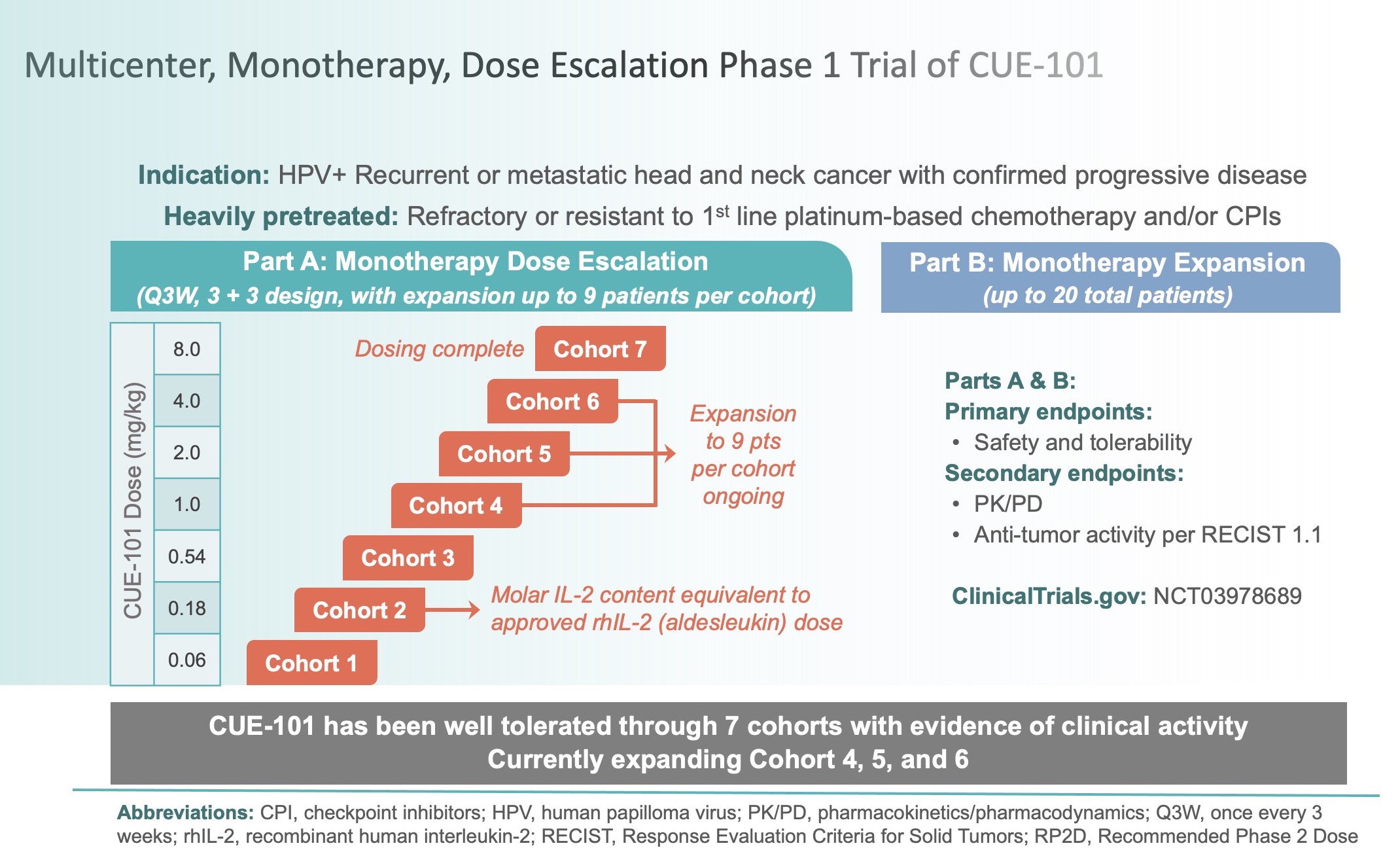
Clinical Trials Cue Biopharma
Ich Format for a Clinical Trial Protocol Clinical Trial Statistics
Free Clinical Trial Templates Smartsheet
WA Health Research Protocol Template for Clinical Trials
(PDF) Development and Implementation of Clinical Trial Protocol
Clinical Trial Protocol
KHP CTU Protocol Template
Related Post: