Pharma.be Cta Template
Pharma.be Cta Template - Forms and formats recommended by european guidances : Imp return form template : The updated contract template can be used for single site as well as. Web pharma blue 50 % c00 m80 y65 k00 r205 g80 b78 pharma red 50 % c00 m40 y100 k00 r230 g165 b2 5 pharma yellow 50 % c50 m00 y80 k00 r160 g195 b9 pharma. Web similarly, if a cro provides its template cta for use by a sponsor, a sponsor should have that template reviewed by sponsor’s counsel. Web european union (eu) pharmaceutical legislation known as the clinical trials regulation entered into application on 31 january 2022. Web the investigator must submit a clinical trial application (‘cta’) to a fully accredited ethics committee and to minister (with delegated authority to the famhp). Web health canada is pleased to announce the release of the guidance document quality (chemistry and manufacturing) guidance: Web a clinical trial application (cta) is a submission to the competent national regulatory authority (ies) for obtaining authorization to conduct a clinical trial in a specific country. Packing of imp form template : Web the suite of model site agreements are supported by guidance which sets out the aims and provides details on how the agreement should be used in the development of contracts for clinical research sponsored by pharmaceutical, biopharmaceutical or medical technology companies. Web pharma.be cta template_1st april 2019_final 41 draft agreement for clinical trials study protocol n° [insert number] center:. Web pharmacy accountability form template. Web scope of public consultation. Web we can now announce that the cta contract template for clinical trials in pharmaceuticals has been updated. Packing of imp form template : Web the suite of model site agreements are supported by guidance which sets out the aims and provides details on how the agreement should be used. Cta request form + ecs form. Imp return form template : Web any other person exercising a profession referred to in all applicable laws as defined in article 1.3 and who is qualified for conducting a study, responsible for the conduct and. Web health canada is pleased to announce the release of the guidance document quality (chemistry and manufacturing) guidance:. Web a clinical trial application (cta) is a submission to the competent national regulatory authority (ies) for obtaining authorization to conduct a clinical trial in a specific country. Web similarly, if a cro provides its template cta for use by a sponsor, a sponsor should have that template reviewed by sponsor’s counsel. Forms and formats recommended by european guidances :. Web the transcelerate clinical content & reuse (cc&r) team launched the first edition of the common protocol template (cpt) in 2016 for use in all phases of interventional clinical. Imp return form template : In europe, for all member states. Web scope of public consultation. Web health canada is pleased to announce the release of the guidance document quality (chemistry. In europe, for all member states. Investigator imp accountability form : It aims to ensure the eu offers an attractive. Cta request form + ecs form. Web accelerated clinical trial agreement (acta) the accelerated clinical trial agreement (acta) was prepared with the intent to facilitate relationships with industry sponsors. Web overview of cta templates ref. Web pharmacy accountability form template. In europe, for all member states. Web pharma.be and deloitte collaborated to produce a white paper on innovative clinical trials in belgium, highlighting the opportunities and challenges of this emerging field. Web a clinical trial application (cta) is a submission to the competent national regulatory authority (ies) for obtaining. Web health canada is pleased to announce the release of the guidance document quality (chemistry and manufacturing) guidance: Web a clinical trial application (cta) is a submission to the competent national regulatory authority (ies) for obtaining authorization to conduct a clinical trial in a specific country. Web pharma.be cta template_1st april 2019_final 41 draft agreement for clinical trials study protocol. Web clinical trial applications (ctas) the following section provides the requirements for a cta involving the use of pharmaceutical, biological, and radiopharmaceutical drugs. Imp return form template : Web pharmacy accountability form template. In order to collect comments and suggestions for improvements, the aifa launched a public consultation on the two draft cta. Web a clinical trial application (cta) is. Web any other person exercising a profession referred to in all applicable laws as defined in article 1.3 and who is qualified for conducting a study, responsible for the conduct and. Web the suite of model site agreements are supported by guidance which sets out the aims and provides details on how the agreement should be used in the development. Cta request form + ecs form. It aims to ensure the eu offers an attractive. Web we can now announce that the cta contract template for clinical trials in pharmaceuticals has been updated. Forms and formats recommended by european guidances : Web accelerated clinical trial agreement (acta) the accelerated clinical trial agreement (acta) was prepared with the intent to facilitate relationships with industry sponsors. Web european union (eu) pharmaceutical legislation known as the clinical trials regulation entered into application on 31 january 2022. Web pharmacy accountability form template. In order to collect comments and suggestions for improvements, the aifa launched a public consultation on the two draft cta. Web clinical trial applications (ctas) the following section provides the requirements for a cta involving the use of pharmaceutical, biological, and radiopharmaceutical drugs. Web health canada is pleased to announce the release of the guidance document quality (chemistry and manufacturing) guidance: Web the transcelerate clinical content & reuse (cc&r) team launched the first edition of the common protocol template (cpt) in 2016 for use in all phases of interventional clinical. Web how does pharma.be contribute to the development and innovation of clinical trials in belgium and europe? Web overview of cta templates ref. Find out in this presentation that highlights the challenges,. Web in each ms, for ethics committee and the nca. Imp return form template : In europe, for all member states. Packing of imp form template : Web pharma blue 50 % c00 m80 y65 k00 r205 g80 b78 pharma red 50 % c00 m40 y100 k00 r230 g165 b2 5 pharma yellow 50 % c50 m00 y80 k00 r160 g195 b9 pharma. Web the investigator must submit a clinical trial application (‘cta’) to a fully accredited ethics committee and to minister (with delegated authority to the famhp).Editable CTA Canva Templates Bundle The Lead Lady
Pharmacy Business Card Template PSD, Ai & Vector BrandPacks
Pharmaceutical Animated HTML5 Banner Ad Templates With Scrollable ISI
Pharmaceutical Sales Account Plan Template Google Docs, Word, Apple
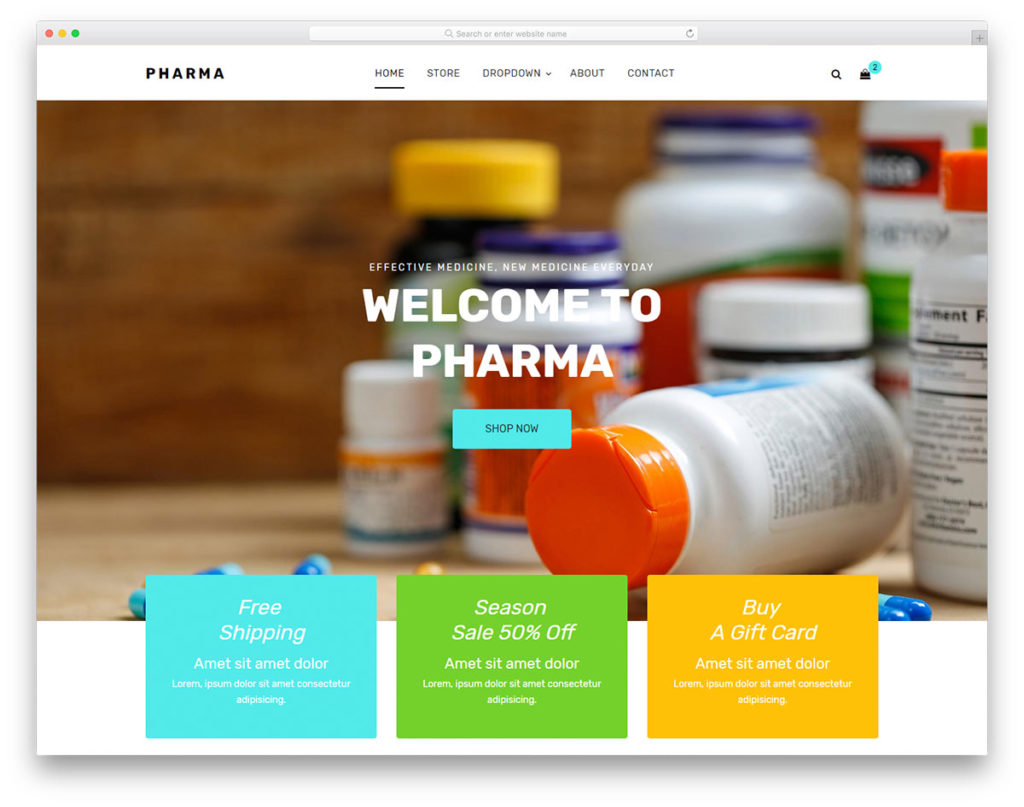
Pharma Free Pharmacy Website Template 2021 Colorlib
Pharma a Free Pharmacy Website Template Best Free HTML/CSS Templates
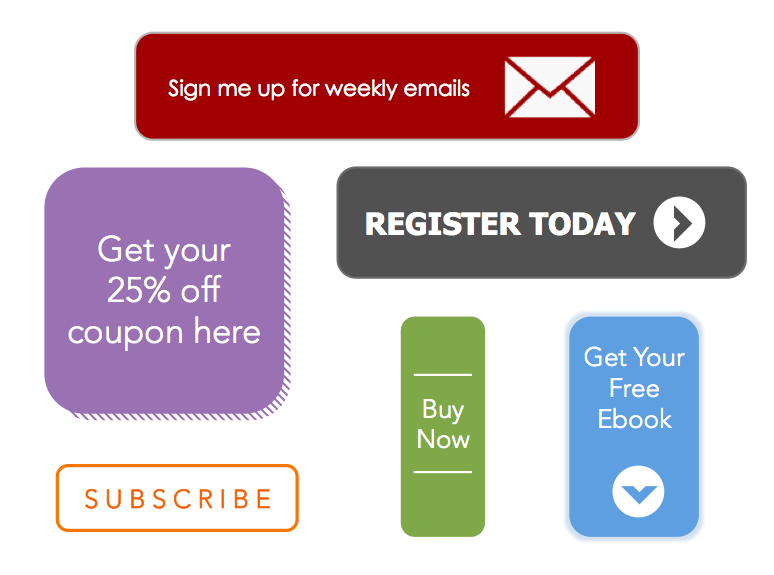
50 Free CalltoAction Templates to Design Clickable CTAs in PowerPoint
50 Free CalltoAction Templates to Design Clickable CTAs in PowerPoint
A4 Pharmacy Poster Template Psd, Ai & Vector Brandpacks Inside
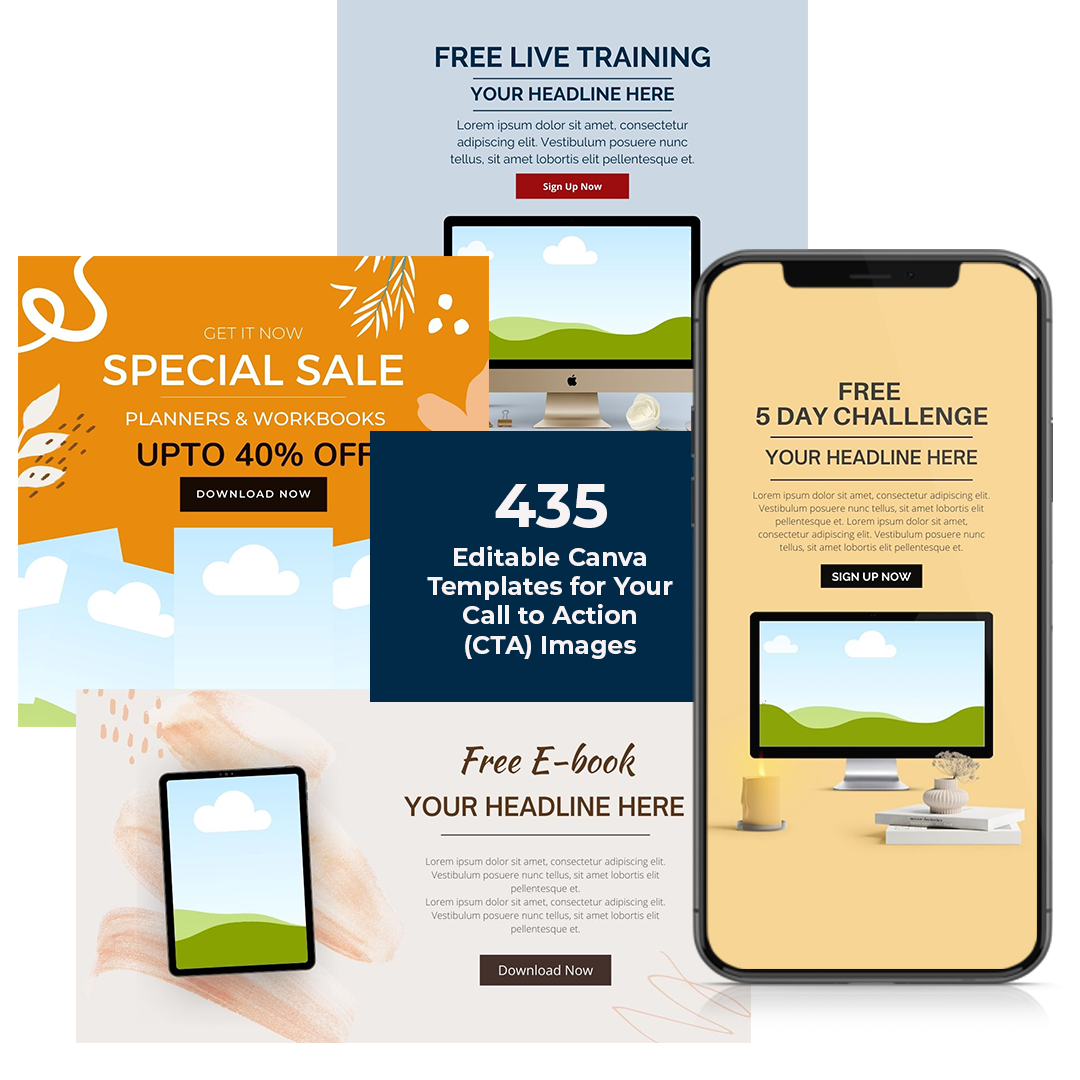
CalltoAction Templates 28 Free CTA Templates [Download Now]
Related Post:






![CalltoAction Templates 28 Free CTA Templates [Download Now]](https://offers.hubspot.com/hubfs/Picture11.png)