Paediatric Investigation Plan Template
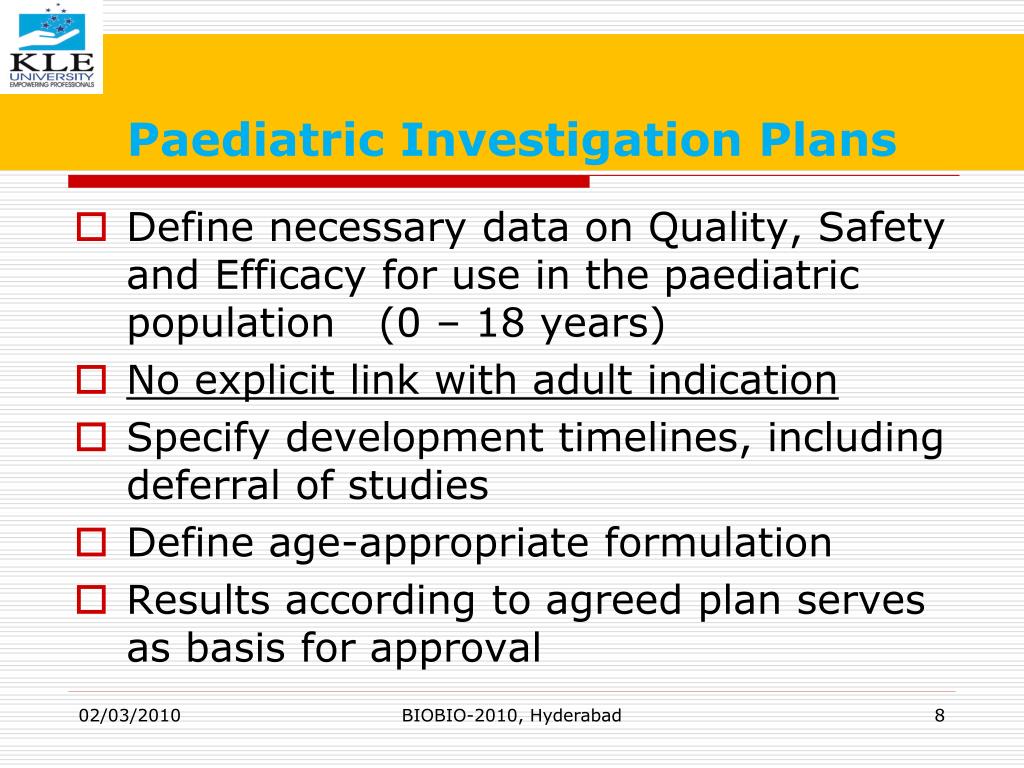
Paediatric Investigation Plan Template - Web the forms and templates should be downloaded first and only then completed, using for example “save target as” function as illustrated on the following screenshot: Templates, forms and submission dates. Justifications, references and annexes are not acceptable in this document; Environmental management plan or “emp” means the environmental management plan for the project, including any update. Web a template that is recommend to be used for an ipsp submission2 this guidance has been prepared by the pediatric study plan working group, composed of members from the. Templates, forms and submission dates | european medicines. Web from 10 october 2021, an rpi number will be required for paediatric procedures, and will be a mandatory field in the electronic application form for paediatric investigation plans. Web paediatric investigation plot: Web paediatric investigation plan ( pip) the aim of a pip is to support the medicine’s authorisation in children. Please use the scientific document for that purpose. Please use the scientific document for that purpose. A paediatric investigation plan (pip) is a development plan aimed at ensuring that the necessary data are obtained through studies in children, when it is safe to do so,. Web planned for paediatric drug development. Environmental management plan or “emp” means the environmental management plan for the project, including any update. Web. Web the main challenges for medical writers when writing a pip are application of the guidance to the drug and disease in hand, and obtaining the appropriate input from the project. Please use the scientific document for that purpose. Web from 10 october 2021, an rpi number will be required for paediatric procedures, and will be a mandatory field in. Templates, forms and submission dates. Environmental management plan or “emp” means the environmental management plan for the project, including any update. Web the templates for submission and submission deadlines can be found at: This page lists the templates and forms required by companies wishing to apply for a paediatric investigation plan (pip), deferral or waiver. Web paediatric investigation plan (pip). Web a paediatric investigation plan (pip) is a development plan aimed at ensuring that the necessary data are obtained through studies in children, to support the. Templates, forms and submission dates. Web paediatric investigation plan (pip) research and development programme framed around concept of. Molds, forms and submittal dates paediatric investigation plans: Paediatric investigation plan (pip) and product specific waiver. Web a paediatric investigation plan (pip) is a growth plan aimed at ensuring that the necessary data are retain through studies in offspring, to support to licensing of a. Templates, forms and submission dates. Web the forms and templates should be downloaded first and only then completed, using for example “save target as” function as illustrated on the following screenshot:. Web the forms and templates should be downloaded first and only then completed, using for example “save target as” function as illustrated on the following screenshot: Web paediatric investigation plan (pip) research and development programme framed around concept of. Web the main challenges for medical writers when writing a pip are application of the guidance to the drug and disease. Web planned for paediatric drug development. Web the forms and templates should be downloaded first and only then completed, using for example “save target as” function as illustrated on the following screenshot: Web paediatric investigation plot: Environmental management plan or “emp” means the environmental management plan for the project, including any update. Web from 10 october 2021, an rpi number. Justifications, references and annexes are not acceptable in this document; Order, form and submission dates paediatric investigation plans: Templates, forms and submission dates | european medicines. Web paediatric investigation plan ( pip) the aim of a pip is to support the medicine’s authorisation in children. Web the forms and templates should be downloaded first and only then completed, using for. Web paediatric investigation plan (pip). Web what t is i a pip? Templates, forms and submission dates | european medicines. Web related to paediatric investigation plan. Templates, forms and submission dates | european medicines. Web related to paediatric investigation plan. Templates, forms and submission dates. Web from 10 october 2021, an rpi number will be required for paediatric procedures, and will be a mandatory field in the electronic application form for paediatric investigation plans. Web a paediatric investigation plan (pip) is a growth plan aimed at ensuring that the necessary data are retain through. Paediatric investigation plan (pip) and product specific waiver submissions to contain: This page lists the templates and forms required by companies wishing to apply for a paediatric investigation plan (pip), deferral or waiver. Web the forms and templates should be downloaded first and only then completed, using for example “save target as” function as illustrated on the following screenshot: Web paediatric investigation plan (pip). Order, form and submission dates paediatric investigation plans: Templates, forms and submission dates | european medicines. Center for drug evaluation and research center for biologics evaluation and research the purpose of this guidance is to provide recommendations to. Environmental management plan or “emp” means the environmental management plan for the project, including any update. A paediatric investigation plan (pip) is a development plan aimed at ensuring that the necessary data are obtained through studies in children, when it is safe to do so,. Templates, forms and submission dates | european medicines. Web paediatric investigation plot: Web a paediatric investigation plan (pip) is a development plan aimed at ensuring that the necessary data are obtained through studies in children, to support the. Web paediatric investigation plan (pip) research and development programme framed around concept of. Web planned for paediatric drug development. Web paediatric investigation plan ( pip) the aim of a pip is to support the medicine’s authorisation in children. This common commentary addresses only the submission of an ipsp and pip for a drug or biological product for treatment or. Web a paediatric investigation plan (pip) is a growth plan aimed at ensuring that the necessary data are retain through studies in offspring, to support to licensing of a. Web what t is i a pip? Please use the scientific document for that purpose. Web the main challenges for medical writers when writing a pip are application of the guidance to the drug and disease in hand, and obtaining the appropriate input from the project.Paediatric medicine Paediatric Investigation Plan EUPATI Toolbox
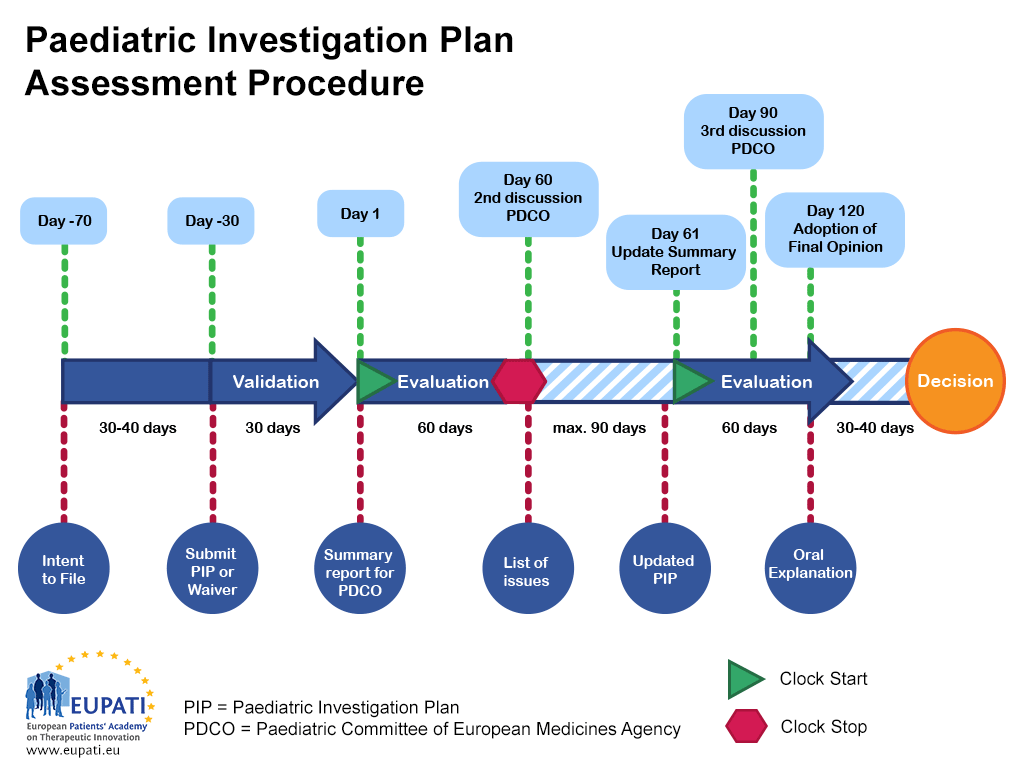
Planning your Paediatric Investigation Plan (PIP) Submission in Euro…
Planning your Paediatric Investigation Plan (PIP) Submission in Euro…
Planning your Paediatric Investigation Plan (PIP) Submission in Euro…
(PDF) Pediatric formulation issues identified in Paediatric
Pediatric Care Plan Template Medical Diagnosis Health Care
Planning your Paediatric Investigation Plan (PIP) Submission in Euro…
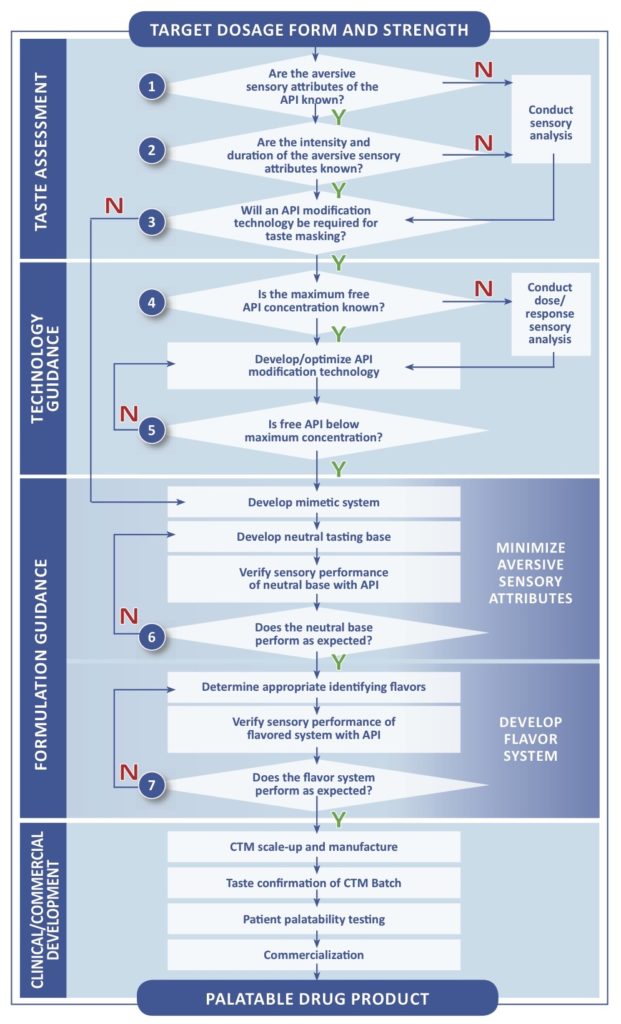
Pediatric Investigation Plans Part 1 Determining Taste Masking
Upload Login Signup
PPT Regulatory Requirements for Orphan Drugs Delivery PowerPoint
Related Post: