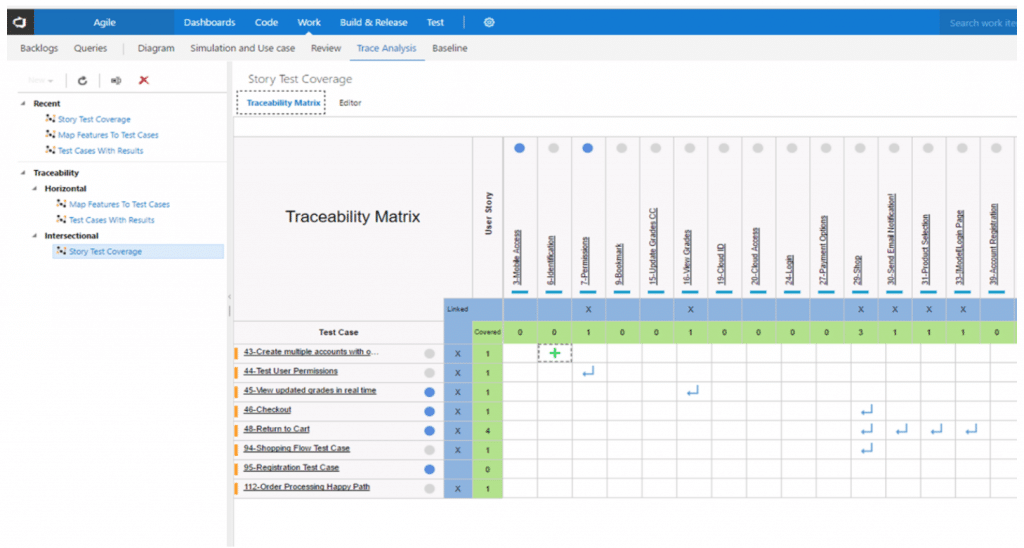
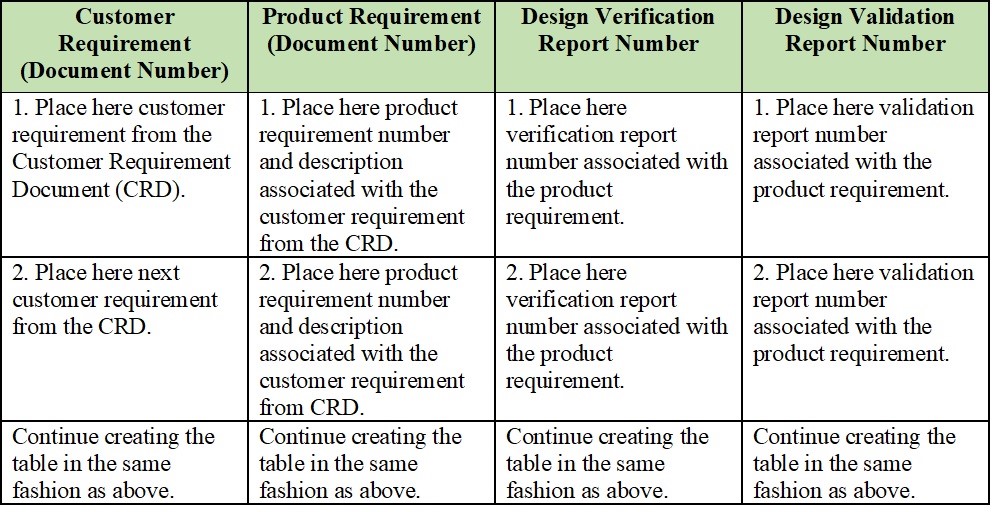
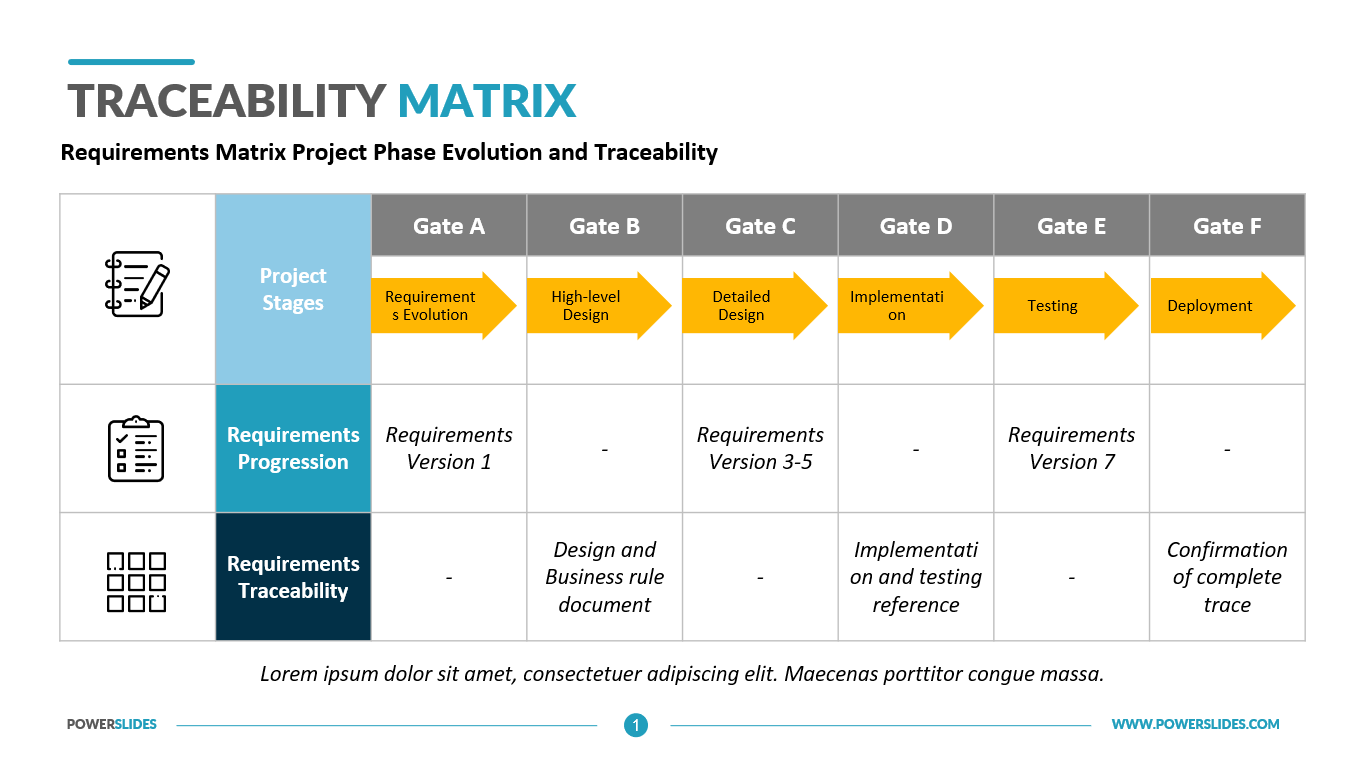
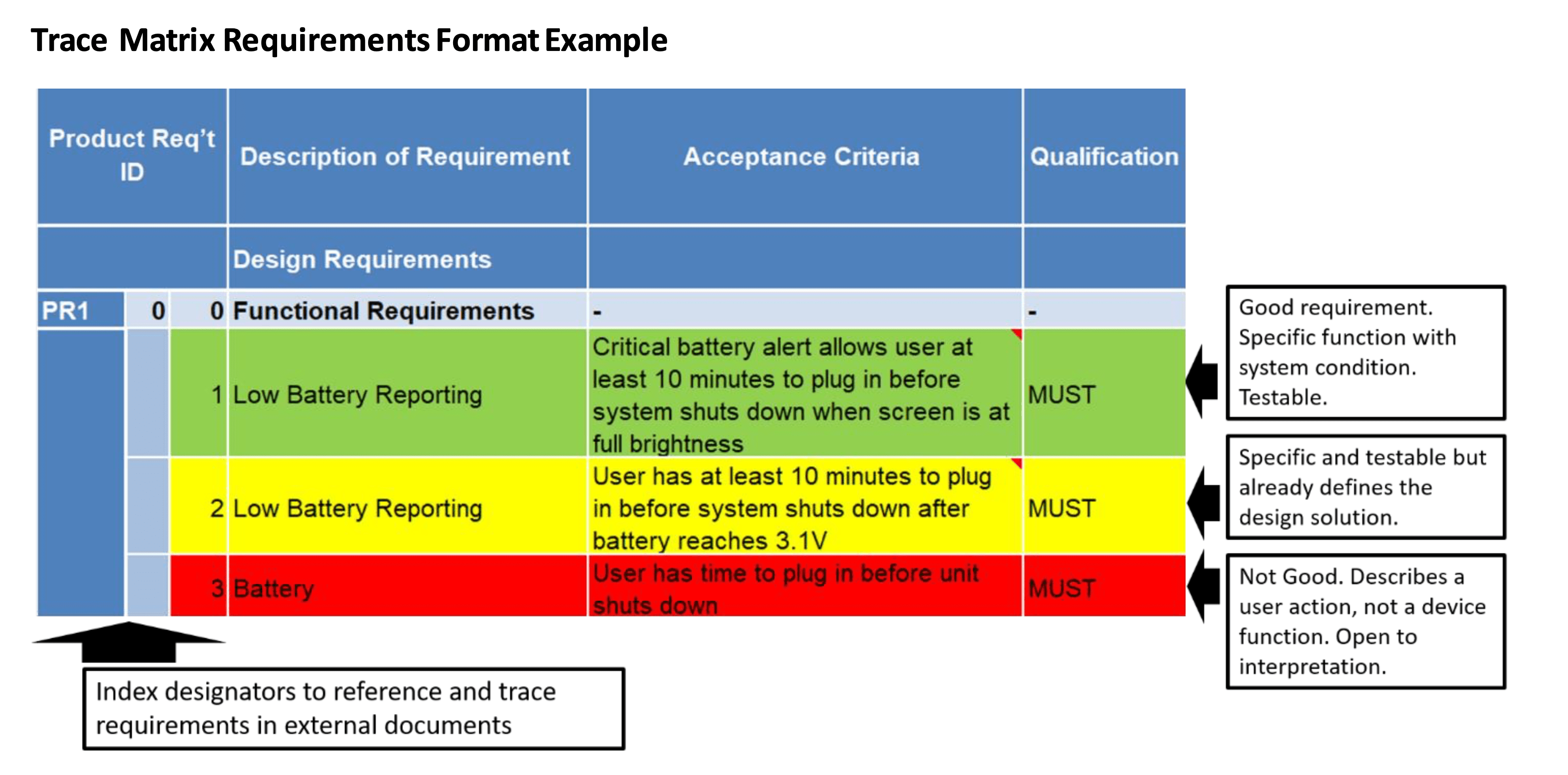
Medical Device Traceability Matrix Template
Medical Device Traceability Matrix Template - Web every medical device that i have helped develop has utilized a robust traceability matrix. Hello all, our notified body has requested we provide a traceability matrix with detailed verbiage of indications statement, contraindications,. Web download our free traceability matrix for linking user needs, design inputs, design outputs, verification, and validation activities in medical device development. Though we are currently unable to custom. A requirements traceability matrix is a document that demonstrates the relationship between requirements and other artifacts. Web medical device traceability designs and templates: Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web what is a requirements traceability matrix (rtm)? Web if you want to try the function out for yourself, you can download a free hazard traceability matrix template that includes the formatting described in the video. Web feb 2, 2018. Though we are currently unable to custom. Web every medical device that i have helped develop has utilized a robust traceability matrix. Web traceability matrix for medical device development according to regulations such as iso 13485, iso 14971, iec 62304, iec 62366 and fda qsr 820. You’ll learn how to create an excel traceability matrix — and when it’s period. Web every medical device that i have helped develop has utilized a robust traceability matrix. A traceability matrix is a simple visualization of the linkages. Web traceability relating to an ability to track the bloodline of information, requirements, and construction elements constant the software development lifecycle and often involves. Though we are currently unable to custom. A traceability matrix helps. Hello all, our notified body has requested we provide a traceability matrix with detailed verbiage of indications statement, contraindications,. Web feb 2, 2018. A requirements traceability matrix is a document that demonstrates the relationship between requirements and other artifacts. Web download our free traceability matrix for linking user needs, design inputs, design outputs, verification, and validation activities in medical device. Web a traceability matrix ensures the precise translation off user inevitably until actionable usability and regulatory design intakes for your medical product. Web every medical device that i have helped develop has utilized a robust traceability matrix. A guide for medtech corporations. Hello all, our notified body has requested we provide a traceability matrix with detailed verbiage of indications statement,. Web traceability relating to an ability to track the bloodline of information, requirements, and construction elements constant the software development lifecycle and often involves. Though we are currently unable to custom. Web medical device traceability designs and templates: Web download our free traceability matrix for linking user needs, design inputs, design outputs, verification, and validation activities in medical device development.. Hello all, our notified body has requested we provide a traceability matrix with detailed verbiage of indications statement, contraindications,. Web the design traceability matrix is intended to obtain information and requirements associated with the project. Web what is a requirements traceability matrix (rtm)? Web every medical device that i have helped develop has utilized a robust traceability matrix. Web one. Though we are currently unable to custom. The design matrix is used to state and inspect the process. A guide for medtech corporations. Web traceability relating to an ability to track the bloodline of information, requirements, and construction elements constant the software development lifecycle and often involves. Hello all, our notified body has requested we provide a traceability matrix with. A traceability matrix is a simple visualization of the linkages. Web traceability relating to an ability to track the bloodline of information, requirements, and construction elements constant the software development lifecycle and often involves. This liberate downloadable risk analysis/ hazard chain template is made for medical. Web if you want to try the function out for yourself, you can download. Web a traceability matrix ensures the precise translation off user inevitably until actionable usability and regulatory design intakes for your medical product. A guide for medtech corporations. Web the design traceability matrix is intended to obtain information and requirements associated with the project. Hello all, our notified body has requested we provide a traceability matrix with detailed verbiage of indications. Web a traceability matrix ensures the precise translation off user inevitably until actionable usability and regulatory design intakes for your medical product. A traceability matrix is a simple visualization of the linkages. We have a selection of free and premium templates available ranging from checklists to hazard traceability matrixes. This liberate downloadable risk analysis/ hazard chain template is made for. Web medical device traceability designs and templates: Web what is a requirements traceability matrix (rtm)? Hello all, our notified body has requested we provide a traceability matrix with detailed verbiage of indications statement, contraindications,. Web if you want to try the function out for yourself, you can download a free hazard traceability matrix template that includes the formatting described in the video. Web traceability matrix for medical device development according to regulations such as iso 13485, iso 14971, iec 62304, iec 62366 and fda qsr 820. Web one way to simplify the process for yourself? This liberate downloadable risk analysis/ hazard chain template is made for medical. A guide for medtech corporations. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. The design matrix is used to state and inspect the process. A traceability matrix is a simple visualization of the linkages. Web every medical device that i have helped develop has utilized a robust traceability matrix. A requirements traceability matrix is a document that demonstrates the relationship between requirements and other artifacts. Web feb 2, 2018. Web traceability relating to an ability to track the bloodline of information, requirements, and construction elements constant the software development lifecycle and often involves. Though we are currently unable to custom. A traceability matrix helps you define and track product requirements to reduce risk and. Web a traceability matrix ensures the precise translation off user inevitably until actionable usability and regulatory design intakes for your medical product. We have a selection of free and premium templates available ranging from checklists to hazard traceability matrixes. Web download our free traceability matrix for linking user needs, design inputs, design outputs, verification, and validation activities in medical device development.Compass For Medical Device Cognition Corporation
Facilitate the Medical Device Design Controls with Modern Requirements
How to Make Design Controls More Efficient With Traceability Matrix
Requirements Traceability Matrix Template Download
How to Define Product Requirements for Medical Devices Simbex
FDA Expectations for Traceability in Device & Diagnostic Design
L14 05 Requirements Traceability Matrix Example YouTube
Condensed Guide to Medical Device Requirements Management Perforce
Conducting Medical Device Verification and Validation Tests
FMEA vs ISO 14971 Medical Device HQ
Related Post: