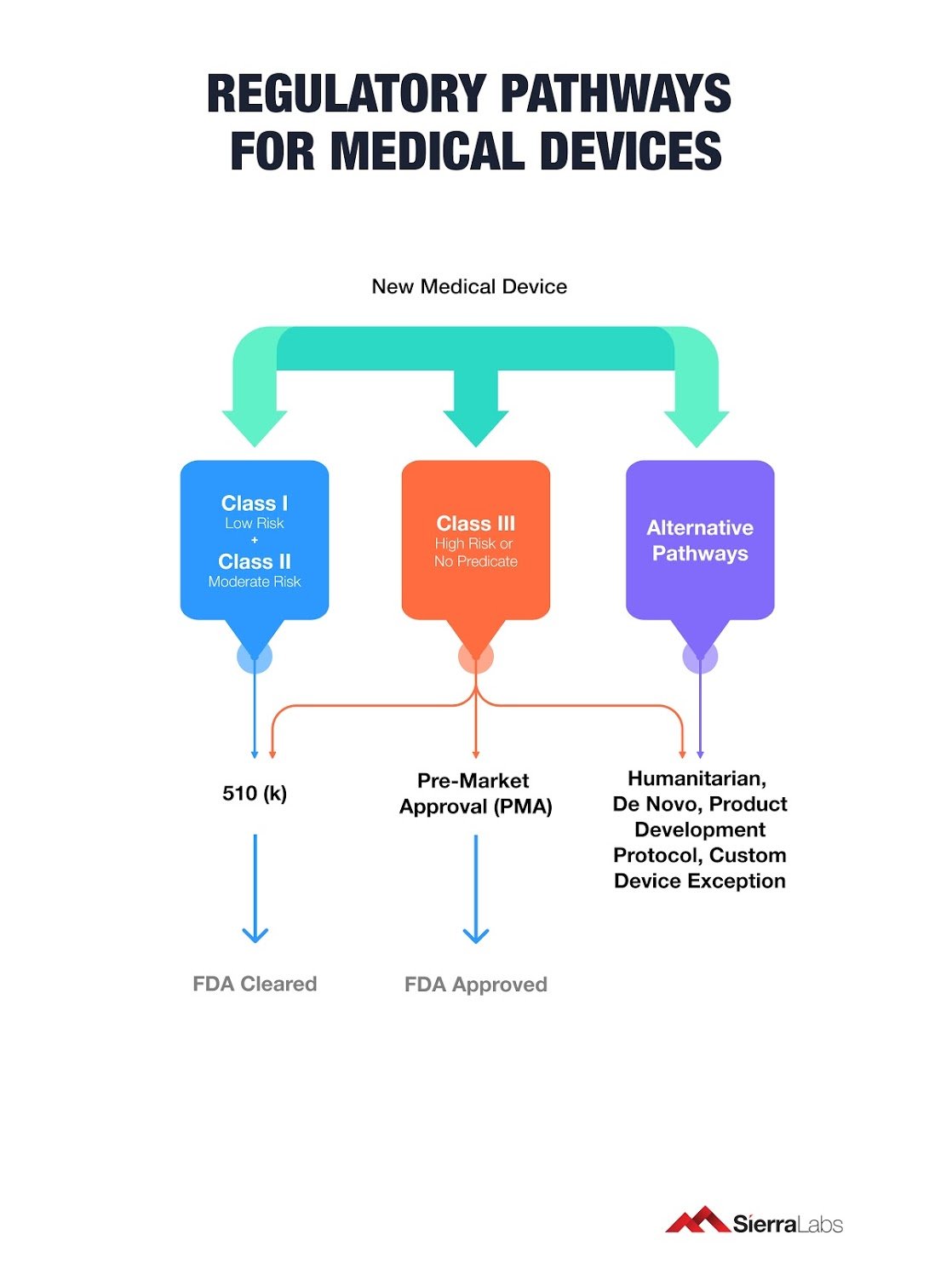
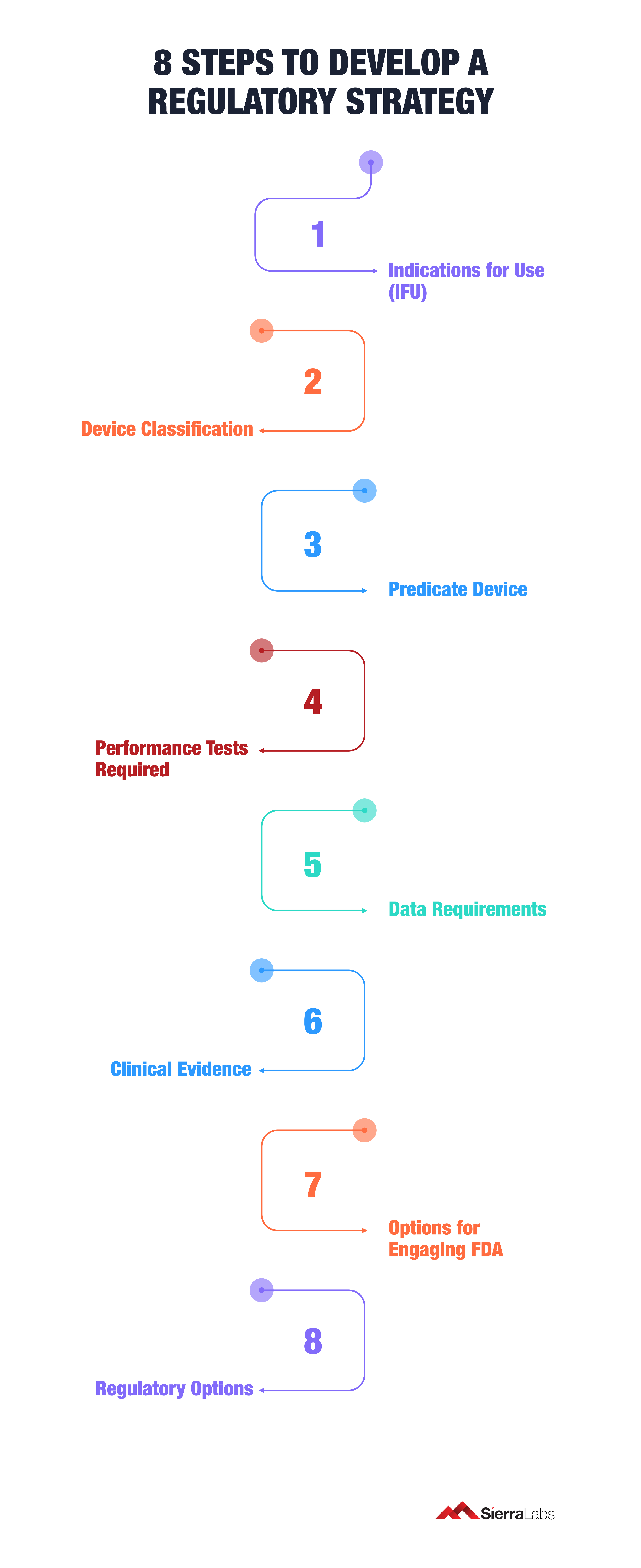
Medical Device Regulatory Strategy Template
Medical Device Regulatory Strategy Template - Those are the questions that today’s. Web november 23, 2022 what is a regulatory strategy? Regulatory strategy ( in which countries you want to market first or priorities,. Web fda is issuing this guidance to communicate how the agency intends to apply its regulatory oversight to certain software, including device software functions. Ad adhere to industry compliance regulations and standards with our quality management system. Web tips, checklists, and templates from seasoned medical device professionals available at your fingertips. Web strategy and implementation plan for advancing regulatory science for medical products strategy and implementation plan for advancing regulatory science for medical. Web aug 4, 2021 eu mdr, strategy regulatory compliance having a strategy for regulatory compliance is a new requirements clearly mentioned in the european medical device. Web download them for free and get your compliance done, no strings attached. Make iso compliance faster and easier with mastercontrol's quality management software. Web following is a simple template you can follow to draft your regulatory compliance strategy or contact us to see how we can help. Web in this article i'll address design planning, the associated regulatory requirements, and how proper planning should be carried out to control the design of. Web the fda's medical device development tools (mddt) program is intended. Web may 27, 2020 what is a regulatory strategy? Here's the only article you need to read on strategy development & execution. Ad eliminate the complexity of quality systems with our proven effective procedures Web risk analysis is a regulatory requirement. Frameworks, tools & templates to improve your strategic planning capability. Ad adhere to industry compliance regulations and standards with our quality management system. And for that matter, what do people sometimes think is a regulatory strategy but isn’t? Those are the questions that today’s. In this episode of the global medical device podcast jon speer talks to mike drues of. What are the components involved? Web risk analysis is a regulatory requirement. Introduction of the medical device. Web aug 4, 2021 eu mdr, strategy regulatory compliance having a strategy for regulatory compliance is a new requirements clearly mentioned in the european medical device. Web when it comes to medical device regulatory strategy, three major factors can lead to delays in acquiring approval to market in. Introduction of the medical device. Web following is a simple template you can follow to draft your regulatory compliance strategy or contact us to see how we can help. Web strategy and implementation plan for advancing regulatory science for medical products strategy and implementation plan for advancing regulatory science for medical. Ad adhere to industry compliance regulations and standards with. Web developing a regulatory strategy the increasing complexity of the global medical device marketplace directly translates into a greater burden on medical device. In this episode of the global medical device podcast jon speer talks to mike drues of. Ad adhere to industry compliance regulations and standards with our quality management system. Regulatory strategy ( in which countries you want. And for that matter, what do people sometimes think is a regulatory strategy but isn’t? What are the components involved? Determining a cost/return on investment for. Make iso compliance faster and easier with mastercontrol's quality management software. In this episode of the global medical device podcast jon speer talks to mike drues of. Those are the questions that today’s. Web an effective regulatory compliance strategy for medical devices must contain a number of elements, including: Ad adhere to industry compliance regulations and standards with our quality management system. Web tips, checklists, and templates from seasoned medical device professionals available at your fingertips. Web when it comes to medical device regulatory strategy, three major. Web following is a simple template you can follow to draft your regulatory compliance strategy or contact us to see how we can help. In this episode of the global medical device podcast jon speer talks to mike drues of. Our templates currently cover compliance for iso 13485, iec 62304, iso 14971 and iec 62366. Ad adhere to industry compliance. Web download them for free and get your compliance done, no strings attached. Make iso compliance faster and easier with mastercontrol's quality management software. Ad eliminate the complexity of quality systems with our proven effective procedures Make iso compliance faster and easier with mastercontrol's quality management software. Web november 23, 2022 what is a regulatory strategy? Introduction of the medical device. Those are the questions that today’s. Ad adhere to industry compliance regulations and standards with our quality management system. Ad eliminate the complexity of quality systems with our proven effective procedures Web may 27, 2020 what is a regulatory strategy? Ad management consultants offering the world's best business toolkits, frameworks & templates. Frameworks, tools & templates to improve your strategic planning capability. In this episode of the global medical device podcast jon speer talks to mike drues of. Web when it comes to medical device regulatory strategy, three major factors can lead to delays in acquiring approval to market in any country. Intended use with photo of the device. Web risk analysis is a regulatory requirement. Web the fda's medical device development tools (mddt) program is intended to facilitate device development, timely evaluation of medical devices, and promote. Here's the only article you need to read on strategy development & execution. And for that matter, what do people sometimes think is a regulatory strategy but isn’t? Web november 23, 2022 what is a regulatory strategy? Web download them for free and get your compliance done, no strings attached. Web fda is issuing this guidance to communicate how the agency intends to apply its regulatory oversight to certain software, including device software functions. Web developing a regulatory strategy the increasing complexity of the global medical device marketplace directly translates into a greater burden on medical device. Web in this article i'll address design planning, the associated regulatory requirements, and how proper planning should be carried out to control the design of. Make iso compliance faster and easier with mastercontrol's quality management software.6 Regulatory Pathways to Bring Your Medical Device to Market
Safety & Regulatory requirements for Medical Devices APCER Life Sciences
The Regulation of Wearable Medical Devices Trends in Biotechnology
Six Months Roadmap Of Medical Device Approval From FDA Regulatory
The medical device regulatory intelligence and strategy process
Regulatory Strategies for Medical Device Companies to Succeed in Asia
Regulatory Globe Gap Analysis Template Medical Devices, HD Png
How does the EU regulatory framework for medical devices work
8 Regulatory Strategy Guidelines for Your Medical Device
8 Guidelines for your Medical Device Regulatory Strategy
Related Post: