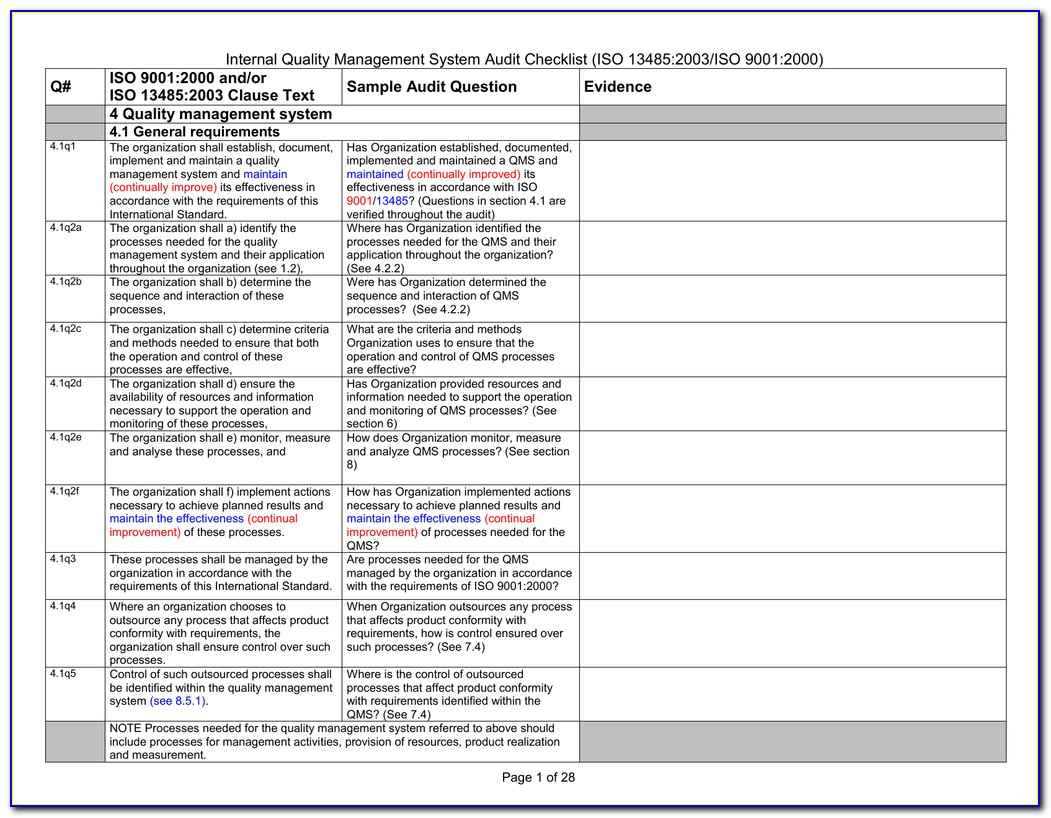
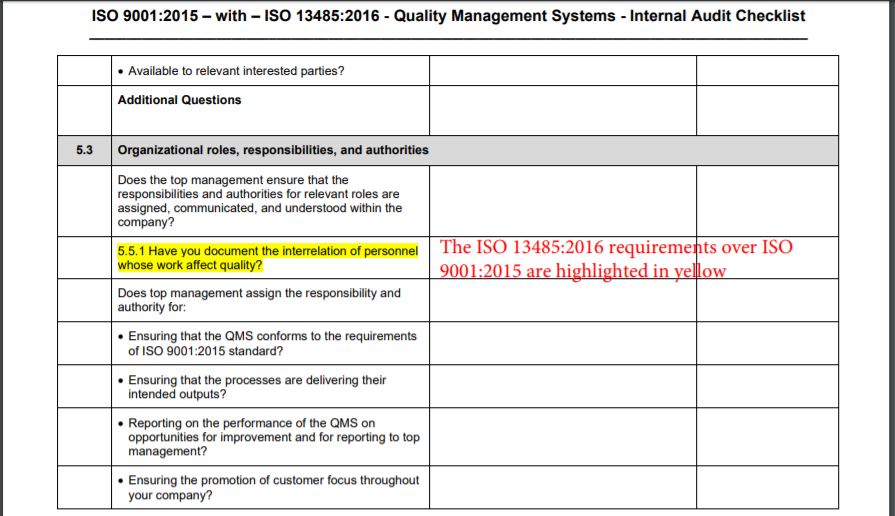
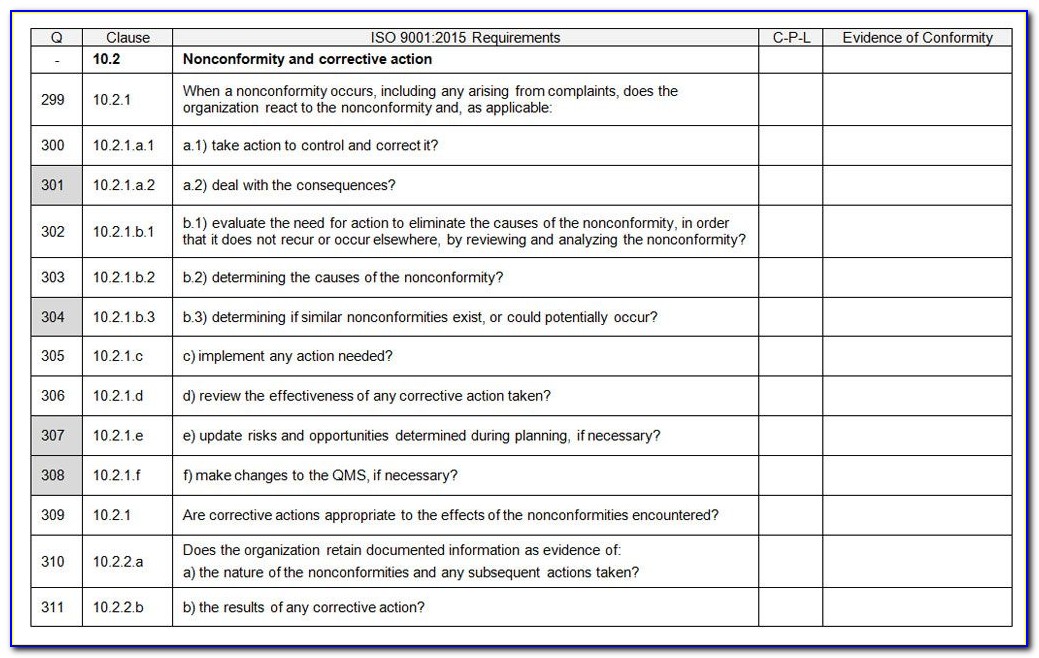
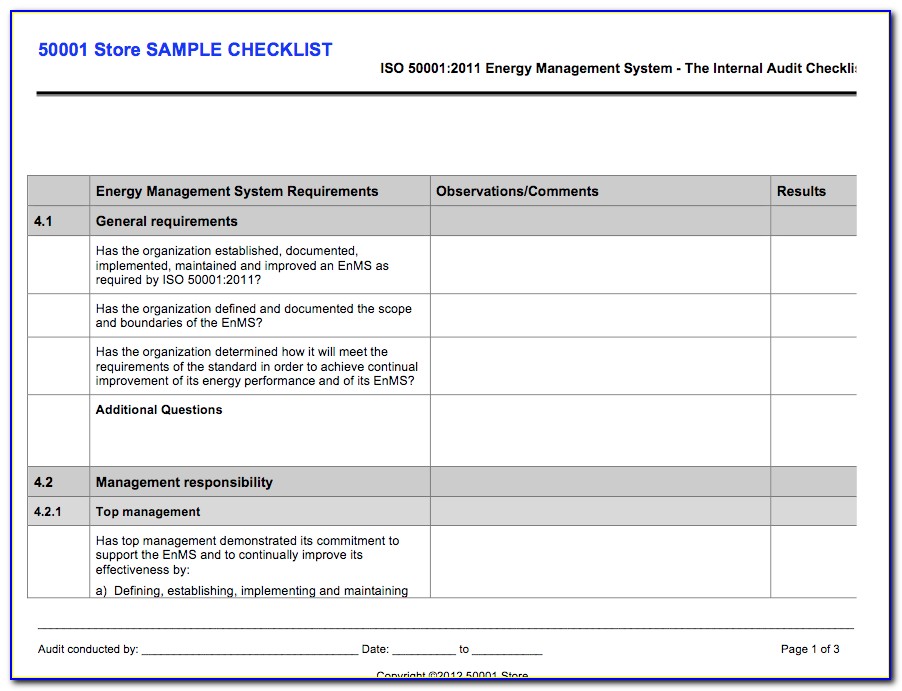
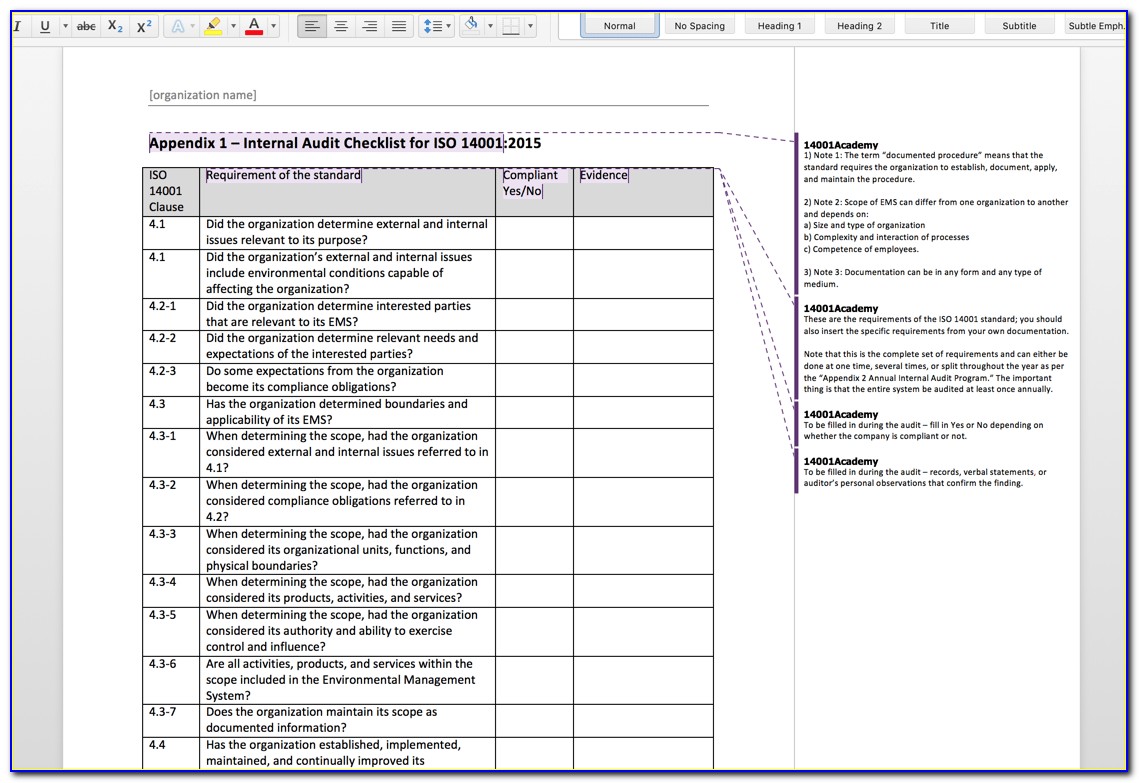
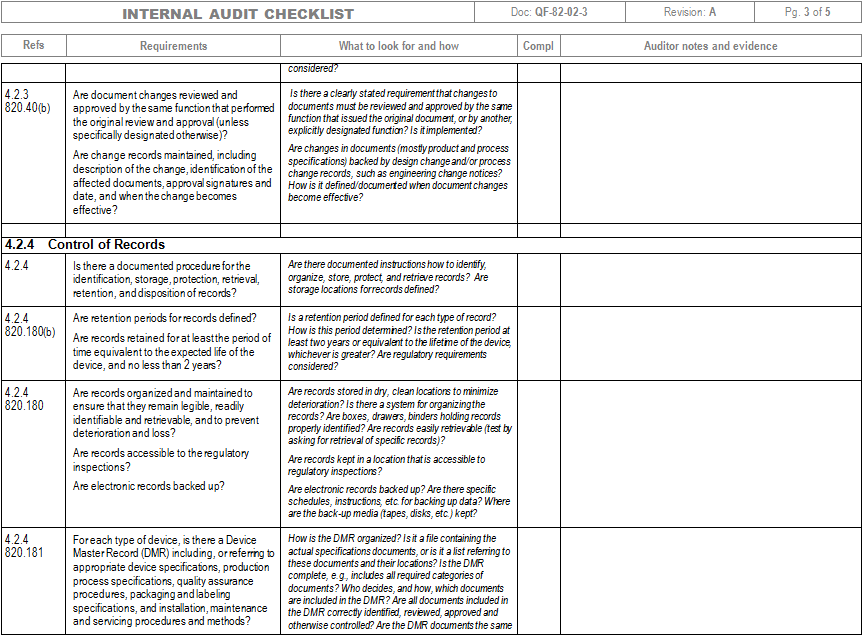
Iso 13485 Audit Checklist Template
Iso 13485 Audit Checklist Template - Ad automate & standardize processes with our quality management software & achieve compliance. Web iso 13485 lays out the broad quality requirements for the modern medical device quality management system. Ad let us help you get to market faster with our efficient quality system templates Web looking to make your own or download iso 13485 audit checklist template to view all the tasks required and tick off the tasks when completed? Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. These templates can include forms for. Make your business fitter, faster & stronger with our quality management system software. Web one way to simplify this process is by using audit checklist templates. Web objective parties conduct internal audits. Has implemented 4.2.1(a), quality 5.1(b), policy (c), 5.3, and 5.4.1) quality objectives? Firm has established quality audit procedures and conducts. Web these templates provide a structured approach to conducting internal audits and ensure that all necessary requirements are met. Web download free template. The checklist is best used by trained and practicing. Web objective parties conduct internal audits. Web these templates provide a structured approach to conducting internal audits and ensure that all necessary requirements are met. Compliance is critical in the medical device world to ensure safety of the consumers. Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Web iso 13485 quality checklist (mdqms) for organizations providing medical devices. Web updated november 22, 2022 iso 13485 templates dr. Here are all our posts. Internal audits are one of the most important process within a quality management system for medical device manufacturers and having an iso. Web an iso 13485 audit checklist is used by quality managers to determine whether the company's quality management system (qms) is compliant with the. These templates help ensure that nothing falls through the cracks during an audit and provide a. Web forms and checklists are used to record data, capture information, and facilitate compliance with iso 13485 requirements. An iso 13485 audit checklist is used for mdsap certification to determine if the organization’s qms is aligned with the iso. View or download free iso. Familiarize yourself with the checklist to understand the questions or criteria it includes. The quality manual defines the scope of your qms and its procedures. Ad deepen your knowledge of the 13485 standards and learn how we can help with compliance. Web pdf template, an iso 13485 audit checklist is utilized by quality managers to determine if the organization’s qms. By leveraging these tools effectively, companies. Web the auditor will use pro qc’s standard audit checklist as a tool that thoroughly assesses the device manufacturer’s quality management system against the. Ensure that you have a clear understanding of the requirements of iso 13485 to effectively use it, too. Ad automate & standardize processes with our quality management software & achieve. Web an iso 13485 audit checklist is used by quality managers to determine whether the company's quality management system (qms) is compliant with the iso 13485:2016. Has implemented 4.2.1(a), quality 5.1(b), policy (c), 5.3, and 5.4.1) quality objectives? Ad deepen your knowledge of the 13485 standards and learn how we can help with compliance. Web download free template. Ad automate. Web pdf template, an iso 13485 audit checklist is utilized by quality managers to determine if the organization’s qms is aligned with the iso 13485:2016 standard. Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Ad deepen your knowledge of the 13485 standards and learn how we can help with compliance. Use this. These templates help ensure that nothing falls through the cracks during an audit and provide a. View or download free iso 13485. Web looking to make your own or download iso 13485 audit checklist template to view all the tasks required and tick off the tasks when completed? The checklist is best used by trained and practicing. Has implemented 4.2.1(a),. Web use this iso 13485 internal audit checklist template to determine whether the company's quality management system (qms) is compliant with the iso standards. Has implemented 4.2.1(a), quality 5.1(b), policy (c), 5.3, and 5.4.1) quality objectives? By leveraging these tools effectively, companies. The quality manual defines the scope of your qms and its procedures. Compliance is critical in the medical. Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. The quality manual defines the scope of your qms and its procedures. Ensure that you have a clear understanding of the requirements of iso 13485 to effectively use it, too. Web download free template. By leveraging these tools effectively, companies. Web forms and checklists are used to record data, capture information, and facilitate compliance with iso 13485 requirements. Compliance is critical in the medical device world to ensure safety of the consumers. Web apr 5, 2021 internal audit. Web pdf template, an iso 13485 audit checklist is utilized by quality managers to determine if the organization’s qms is aligned with the iso 13485:2016 standard. Here are all our posts. Web an iso 13485 audit checklist is used by quality managers to determine whether the company's quality management system (qms) is compliant with the iso 13485:2016. Ad deepen your knowledge of the 13485 standards and learn how we can help with compliance. Web iso 13485 quality checklist (mdqms) for organizations providing medical devices and related services, iso 13485:2016 specifies requirements for a quality management. Web looking to make your own or download iso 13485 audit checklist template to view all the tasks required and tick off the tasks when completed? Compliance is critical in the medical device world to ensure safety of the consumers. Web updated november 22, 2022 iso 13485 templates dr. Web one way to simplify this process is by using audit checklist templates. An iso 13485 audit checklist is used for mdsap certification to determine if the organization’s qms is aligned with the iso. Web based on iso 9001:2015, iso 45001:2018, and iso 14001:2015 standards, you can use this audit plan template to conduct an internal audit of your systems as. Ad let us help you get to market faster with our efficient quality system templatesISO 13485 Quality Management and Document Control Software Internal
Free ISO 13485 Audit Checklists SafetyCulture
Iso 13485 Audit Checklist Template
Iso 13485 Audit Checklist 2016 doctorazgard
Iso 13485 internal audit checklist advfaher
Iso 13485 audit checklist
Iso 13485 Internal Audit Schedule Template Gambaran
ISO 13485 2016 Internal Auditor Checklist TQS Inc.
Audit Checklist For ISO 13485 Audit Business
Iso 13485 Internal Audit Checklist
Related Post: