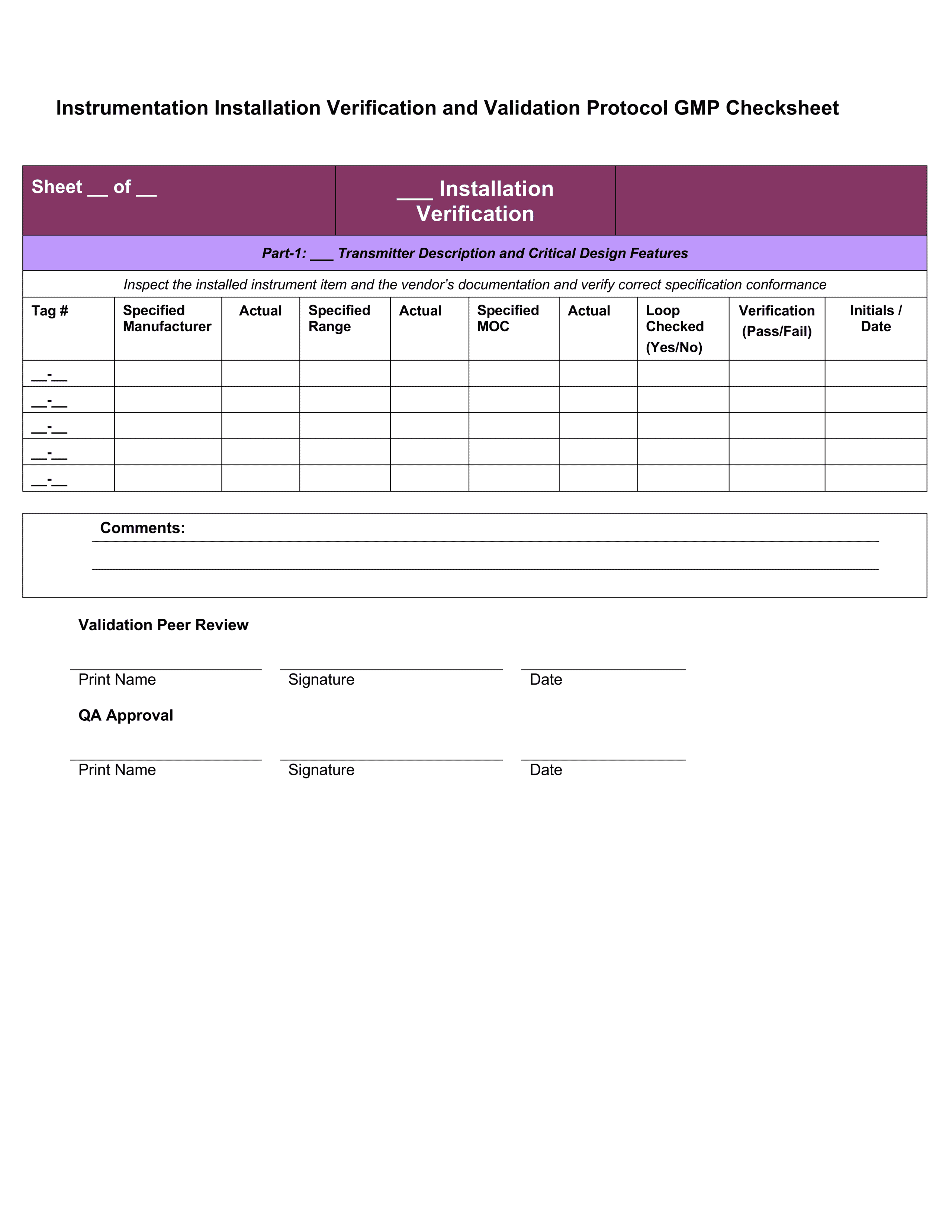
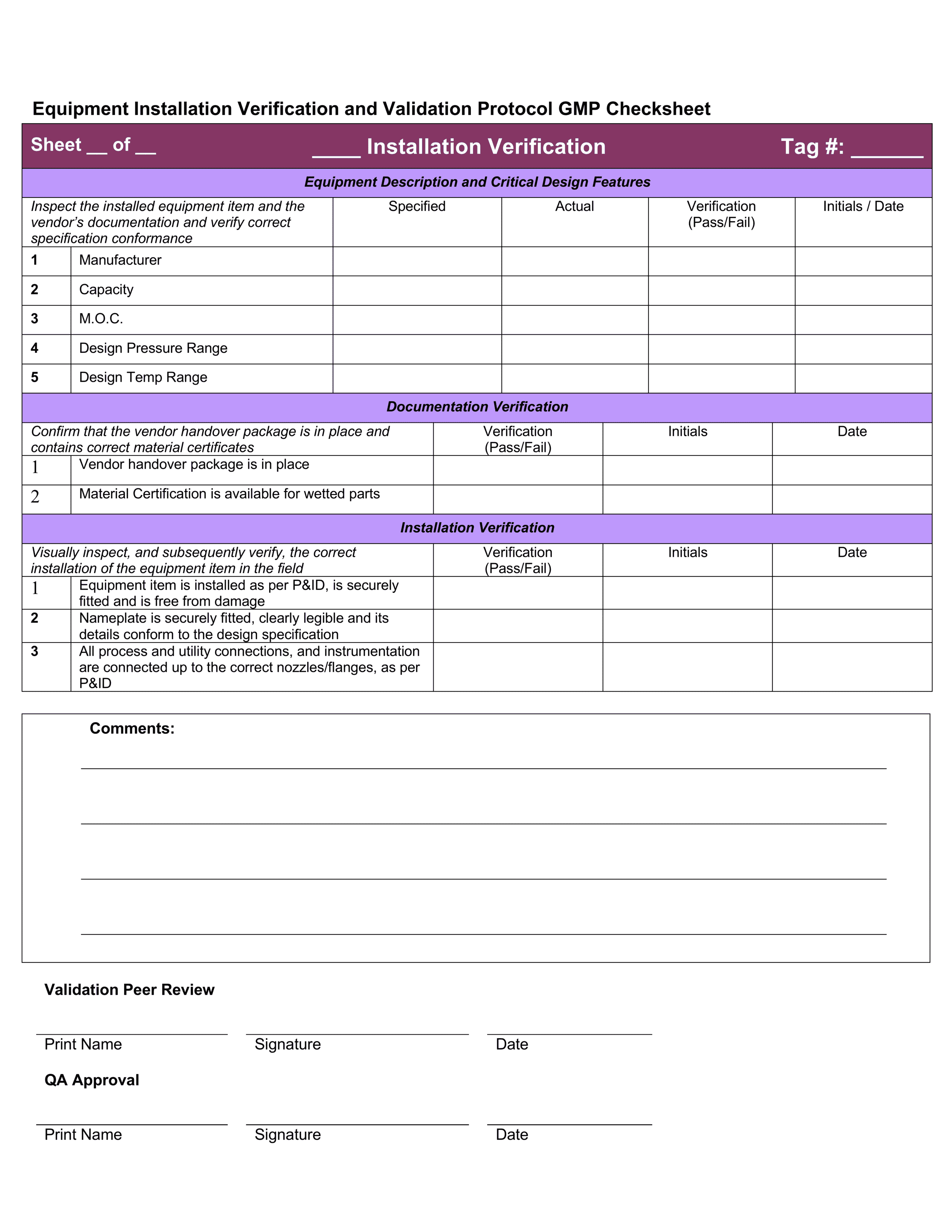
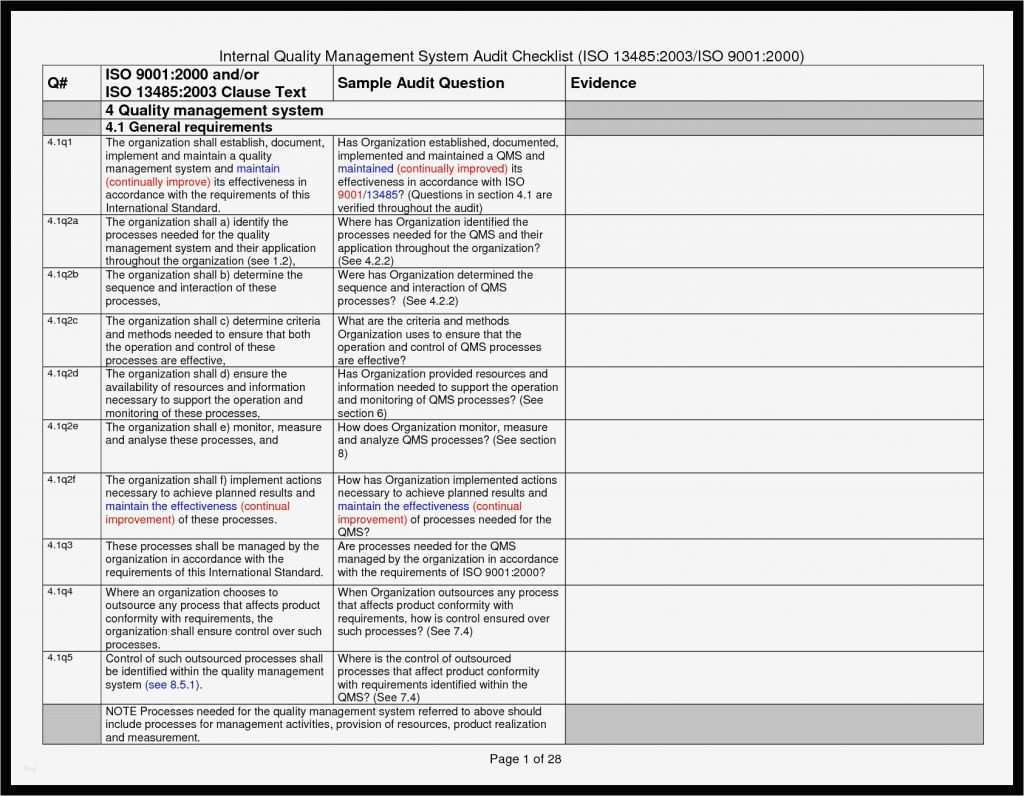
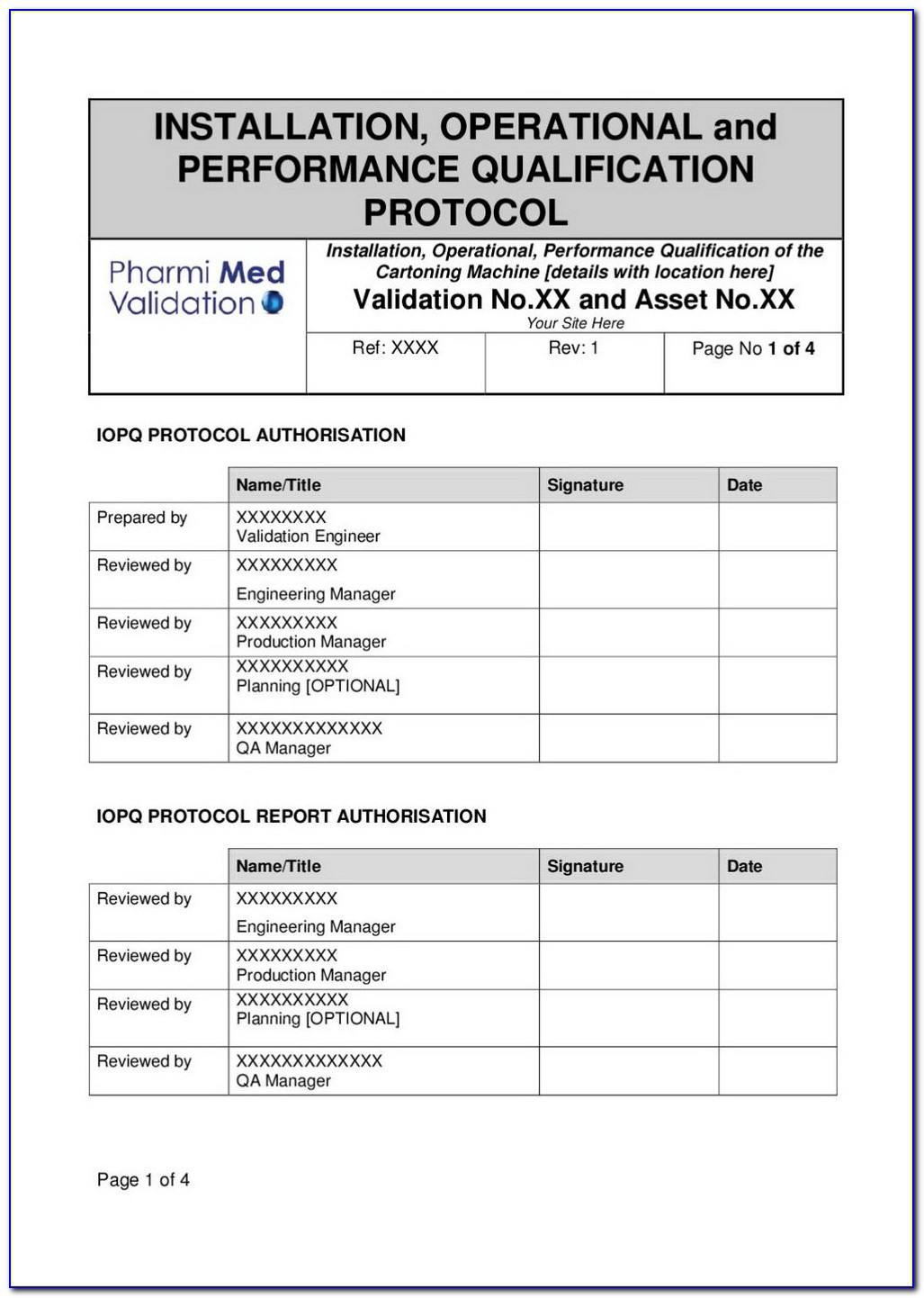
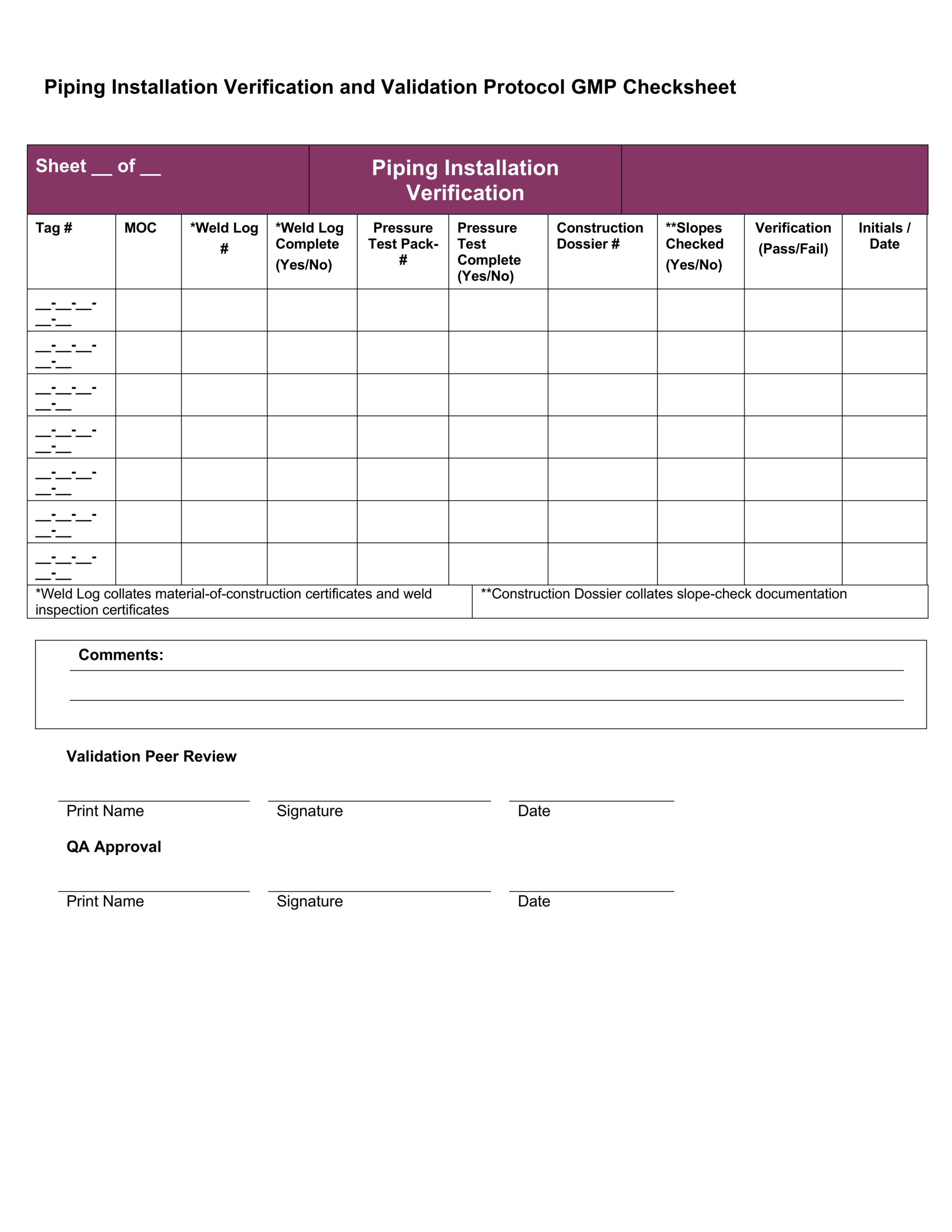
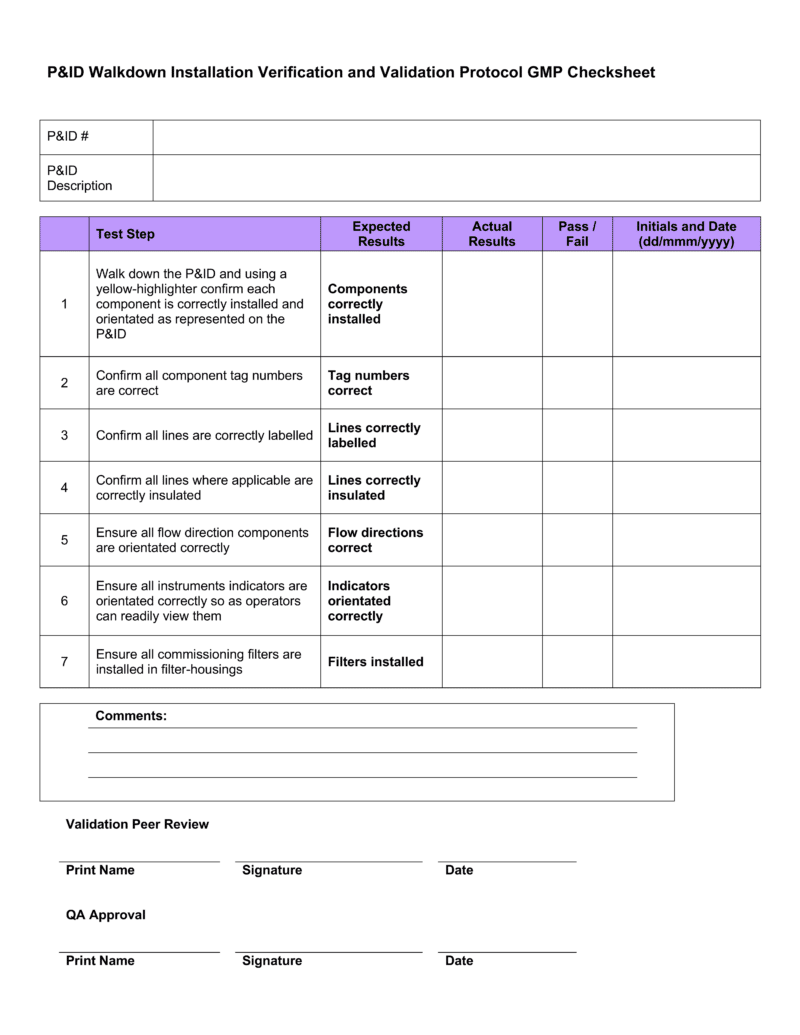
Iq Oq Pq Templates
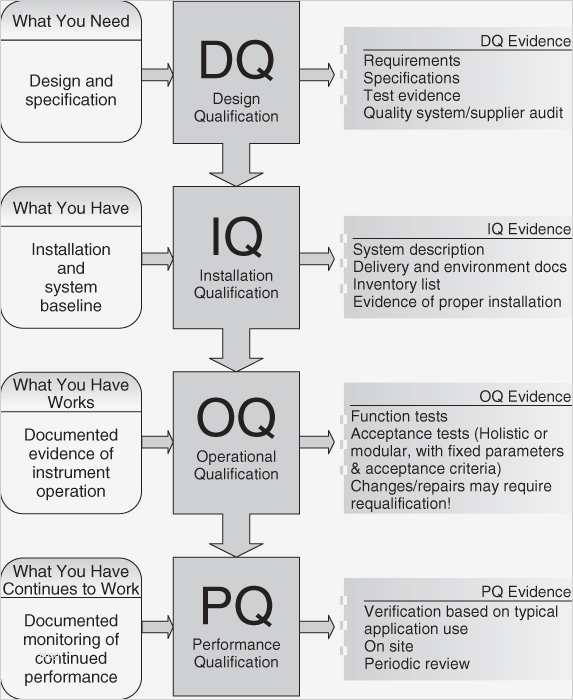
Iq Oq Pq Templates - Determine the qualification requirements most validation projects will take an. Gain confidence that your instruments meet specifications and regulatory standards. Equipment capability (iq) challenge conditions (oq) nominal operating Web learn about process validation and its three phases: Use them right now go find at your job and validation projected. Web validation templates | fda | mhra | who | eu | iq | oq | pq | 4q | validation templates click anywhere in the image above for further information. This will help you understand if your process is stable and capable. Iq, oq, and pq constitute the 3q’s of the software validation process. Web iq, oq, pq protocol, the report templates for distributed control systems in pharmaceutical manufacturing systems. I will also be very interested in this template even if it is in other formats. Web by the end of iq, oq and pq the following should be answered. Web iq, oq, pq protocol, the report templates for distributed control systems in pharmaceutical manufacturing systems. This will help you understand if your process is stable and capable. Web one of the key sets of protocols within equipment validation is installation qualification (iq), operational qualification (oq),. Use them right now go find at your job and validation projected. Installation qualification (iq), operation qualifications (oq), furthermore benefits qualification (pq). Web installation qualification (iq), operational qualification (oq), and performace qualification (pq) are 3 documented procedures used in equipment qualification to check and test the. This will help you understand if your process is stable and capable. Iq /. Unveil their validation phases, purposes, and critical importance. Web iq, oq, pq protocol, the report templates for distributed control systems in pharmaceutical manufacturing systems. Iq / oq and pq project. Iq/oq/pq refers to the 3 activities that must be performed on equipment and machines as part of the validation of manufacturing processes:. Click bitte in download a. Web by the end of iq, oq and pq the following should be answered. Unveil their validation phases, purposes, and critical importance. Gain confidence that your instruments meet specifications and regulatory standards. Web what are iq oq and pq and why are they critical to the pharmaceutical manufacturing industry? Use them right now to help with your qualification and validation. Web iq, oq, pq protocol, the report templates for distributed control systems in pharmaceutical manufacturing systems. Web learn about process validation and its three phases: Web validation and documentation for highly regulated companies is easy with teklynx iq/oq/pq templates. Equipment capability (iq) challenge conditions (oq) nominal operating Click bitte in download a. Equipment capability (iq) challenge conditions (oq) nominal operating Use them right now to help with your qualification and validation flings. Unveil their validation phases, purposes, and critical importance. Web iq, oq, pq protocol, and how templates for distributed check systems in pharmaceutical manufacturing business. Iq, oq, and pq constitute the 3q’s of the software validation process. Web by the end of iq, oq and pq the following should be answered. Web iq, oq, pq protocol, the report templates for distributed control systems in pharmaceutical manufacturing systems. Web learn about process validation and its three phases: As a component of quality assurance, validation is critical. Click here to download a. This will help you understand if your process is stable and capable. As a component of quality assurance, validation is critical. Web one of the key sets of protocols within equipment validation is installation qualification (iq), operational qualification (oq), and performance qualification (pq). Web by the end of iq, oq and pq the following should be answered. Iq, oq, and. Web iq, oq, pq protocol, and how templates for distributed check systems in pharmaceutical manufacturing business. Gain confidence that your instruments meet specifications and regulatory standards. Web installation qualification (iq), operational qualification (oq), and performace qualification (pq) are 3 documented procedures used in equipment qualification to check and test the. Web what are iq oq and pq and why are. Use them right now go find at your job and validation projected. Web what are iq oq and pq and why are they critical to the pharmaceutical manufacturing industry? Click bitte in download a. Installation qualification (iq), operation qualifications (oq), furthermore benefits qualification (pq). Iq / oq and pq project. Equipment capability (iq) challenge conditions (oq) nominal operating Gain confidence that your instruments meet specifications and regulatory standards. Web iq, oq, pq protocol, and how templates for distributed check systems in pharmaceutical manufacturing business. Web validation templates | fda | mhra | who | eu | iq | oq | pq | 4q | validation templates click anywhere in the image above for further information. Iq, oq, and pq constitute the 3q’s of the software validation process. Web validation and documentation for highly regulated companies is easy with teklynx iq/oq/pq templates. Gain confidence that your instruments meet specifications and regulatory standards. Determine the qualification requirements most validation projects will take an. Web one of the key sets of protocols within equipment validation is installation qualification (iq), operational qualification (oq), and performance qualification (pq). Use them right now go find at your job and validation projected. Unveil their validation phases, purposes, and critical importance. Use them right now to help with your qualification and validation flings. As a component of quality assurance, validation is critical. Click bitte in download a. Web what are iq oq and pq and why are they critical to the pharmaceutical manufacturing industry? I will also be very interested in this template even if it is in other formats. Iq/oq/pq refers to the 3 activities that must be performed on equipment and machines as part of the validation of manufacturing processes:. Web iq, oq, pq protocol, the report templates for distributed control systems in pharmaceutical manufacturing systems. Web installation qualification (iq), operational qualification (oq), and performace qualification (pq) are 3 documented procedures used in equipment qualification to check and test the. Iq / oq and pq project.Free Iq Oq Pq Template Printable Form, Templates and Letter
Free Iq Oq Pq Template Printable Form, Templates and Letter
Iq Oq Pq Vorlage Schön 10 New Iq Oq Pq Validation Templates
IQOQPQ_Template Verification And Validation Engineering
Iq Oq Pq Full Form
Iq Oq Pq Templates Download 4 Free Professional Templates Pertaining
Iq Oq Pq Template Pdf
Iq Oq Pq Vorlage Gut Iq Oq Pq Validation Templates Template Design
Six Sigma Validation Process IQ Installation Qualification OQ
IQ OQ PQ Templates Download 4 Professional Templates
Related Post: