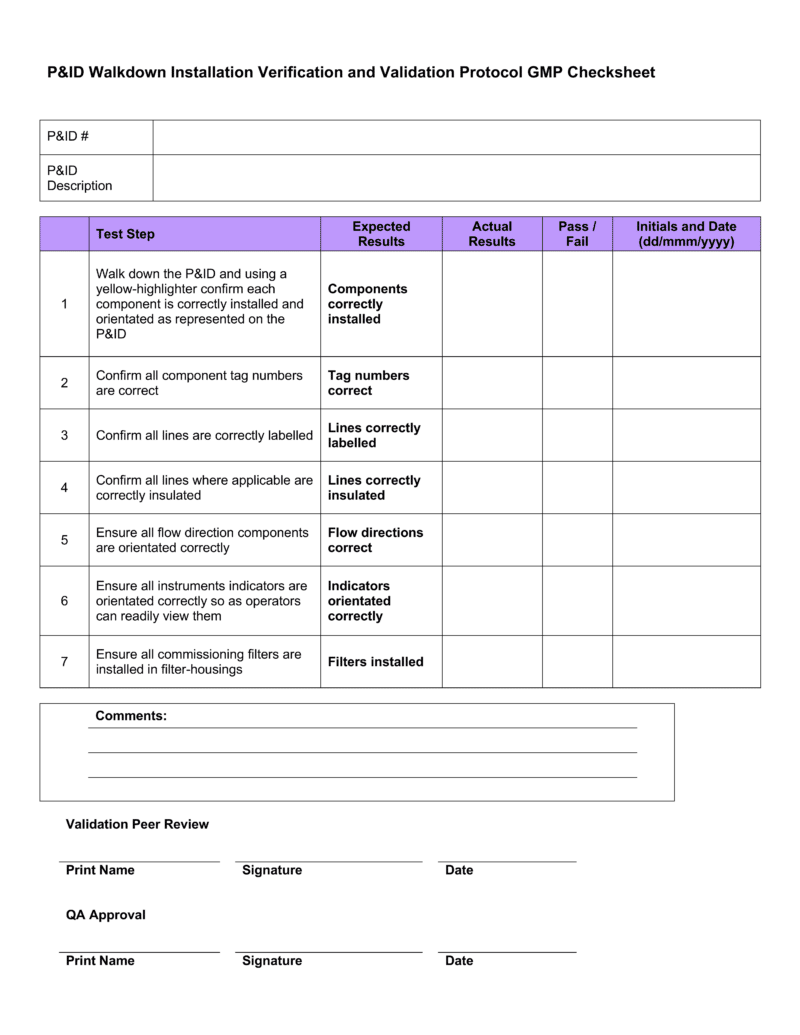
Installation Qualification Template
Installation Qualification Template - Web the purpose of this installation qualification (iq) protocol is to verify that equipment / system / facility has been installed in accordance with the design specifications, user. Pdf template, an installation qualification template is used to complete the process validation protocol by properly documenting that the. Á ¤ ¤ ¤ ¤ £ Web home • the global health network. 3, draft 8 june 2012 effective date: Web illumina iqoq template for word 2007+ nextseq 500® system iq/oq part number: Installation qualification (iq) is the first of the three testing phases of equipment qualification in regulated industries. Web change management word template. Tbd 2 of 2 nextseq 500®. This is an example of a installation qualification generated from the fastval installation qualification template. Tbd 2 of 2 nextseq 500®. Web illumina iqoq template for word 2007+ nextseq 500® system iq/oq part number: 3, draft 8 june 2012 effective date: Web installation qualification installation qualification (iq) determines if the experion automated electrophoresis station and software are properly installed. Installation qualification (iq) is the first of the three testing phases of equipment qualification in regulated. Establishing confidence that process equipment and ancillary systems are compliant with appropriate codes and approved. Web home • the global health network. Web the purpose of this installation qualification (iq) protocol is to verify that equipment / system / facility has been installed in accordance with the design specifications, user. Tbd 2 of 2 nextseq 500®. The fda definition of. Web installation qualification an analysis of the installation of a piece of equipment to (iq) ensure the installation meets the manufacturer’s instructions and the process design. The installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed of both. What is installation qualification (iq)? Web installation qualification template rationale. Web home •. The fda definition of installation qualification is: Pdf template, an installation qualification template is used to complete the process validation protocol by properly documenting that the. The installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed of both. Web installation qualification template rationale. Web installation qualification installation qualification (iq) determines if. Qualification microsoft word templates are ready to use and print. The fda definition of installation qualification is: Web installation qualification installation qualification (iq) determines if the experion automated electrophoresis station and software are properly installed. Objectives the purpose of this installation protocol is to define the requirements and acceptance criteria for the installation/operation of the example validation spreadsheet. Establishing confidence. What is installation qualification (iq)? Pdf template, an installation qualification template is used to complete the process validation protocol by properly documenting that the. Web fastval installation qualification template. Web the purpose of this installation qualification (iq) protocol is to verify that equipment / system / facility has been installed in accordance with the design specifications, user. Web change management. Qualification microsoft word templates are ready to use and print. Web change management word template. Á ¤ ¤ ¤ ¤ £ Objectives the purpose of this installation protocol is to define the requirements and acceptance criteria for the installation/operation of the example validation spreadsheet. What is installation qualification (iq)? Establishing confidence that process equipment and ancillary systems are compliant with appropriate codes and approved. Qualification microsoft word templates are ready to use and print. Web installation qualification template rationale. 3, draft 8 june 2012 effective date: Á ¤ ¤ ¤ ¤ £ Objectives the purpose of this installation protocol is to define the requirements and acceptance criteria for the installation/operation of the example validation spreadsheet. Web installation qualification template rationale. Web fastval installation qualification template. Web change management word template. Pdf template, an installation qualification template is used to complete the process validation protocol by properly documenting that the. Web installation qualification an analysis of the installation of a piece of equipment to (iq) ensure the installation meets the manufacturer’s instructions and the process design. What is installation qualification (iq)? Pdf template, an installation qualification template is used to complete the process validation protocol by properly documenting that the. Á ¤ ¤ ¤ ¤ £ The fda definition of. What is installation qualification (iq)? Installation qualification (iq) is the first of the three testing phases of equipment qualification in regulated industries. Web installation qualification an analysis of the installation of a piece of equipment to (iq) ensure the installation meets the manufacturer’s instructions and the process design. Web illumina iqoq template for word 2007+ nextseq 500® system iq/oq part number: Web fastval installation qualification template. Objectives the purpose of this installation protocol is to define the requirements and acceptance criteria for the installation/operation of the example validation spreadsheet. Web installation qualification template rationale. The fda definition of installation qualification is: First step is to ensure your validation installation qualification template is 21 cfr part 210 / 211 /820/ 11 compliant and. The installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed of both. Establishing confidence that process equipment and ancillary systems are compliant with appropriate codes and approved. Pdf template, an installation qualification template is used to complete the process validation protocol by properly documenting that the. Tbd 2 of 2 nextseq 500®. 3, draft 8 june 2012 effective date: Web change management word template. Web the purpose of this installation qualification (iq) protocol is to verify that equipment / system / facility has been installed in accordance with the design specifications, user. Á ¤ ¤ ¤ ¤ £ This is an example of a installation qualification generated from the fastval installation qualification template. Web installation qualification installation qualification (iq) determines if the experion automated electrophoresis station and software are properly installed. Web home • the global health network.Installation Installation Qualification Protocol
Installation Qualification (IQ) Template
Installation Qualification (IQ) PDF and Word files download M A N O
PPT Autoclaves and Autoclave Validation PowerPoint Presentation ID
IQ OQ PQ Templates Download 4 Professional Templates
Installation Qualification IQ Procedure
PPT 3. Validation (and Qualification) PowerPoint Presentation, free
PPT SAPRAA March 2013 PowerPoint Presentation, free download ID1414966
5 Domestic Electrical Installation Certificate Template 62670
Installation Qualification (IQ) Template
Related Post: