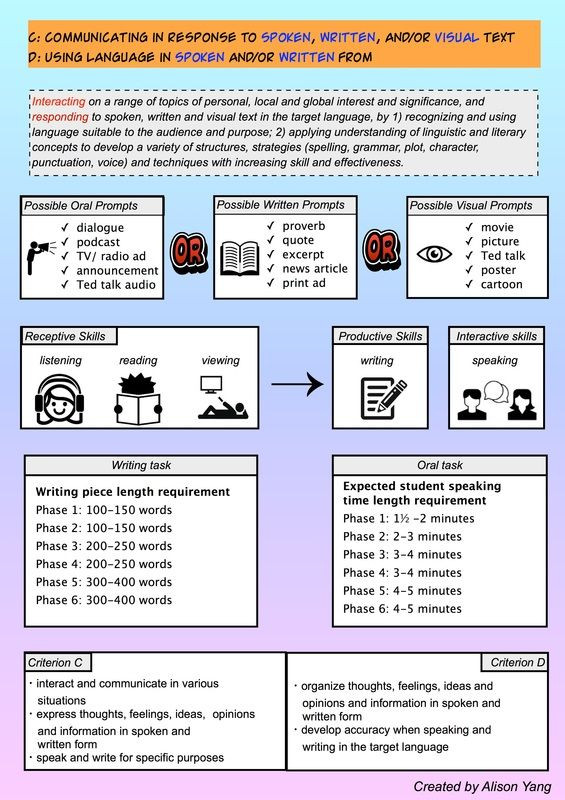
Ib Template
Ib Template - If you have a template to share with your fellow chapter members, or you can't find a template that suits your needs, please contact. Web use this template to set up payments for a loan with principal and interest calculated for you. Moving forward as an ib. Web philosophy of the international baccalaureate programme and the broad learning experience that the ib offers. Web these documents serve to demonstrate the compliance of the investigator, sponsor and monitor with the standards of good clinical practice and with all applicable regulatory. The purpose of the ib is to compile data relevant to studies of the ip in human subject… 06 june 2023 the international baccalaureate® (ib) provides several resources for diploma programme (dp). How to become an ib world school. Web ib should provide a description of the possible risks and adverse drug reactions to be anticipated on the basis of prior experiences with the product under investigation and. Plus, resources to support their use, implementation,. The investigator’s brochure (ib) is a compilation of the clinical and nonclinical data on the investigational product (s) that are relevant to the study of the. Plus, resources to support their use, implementation,. In drug development and medical device development the investigator's brochure (ib) is a comprehensive document summarizing the body of information about an investigational product (ip or study. Web this template presents the sections that comprise the ind application and was derived from fda ind regulations (21crf312.23) and ich good clinical practice guidelines. See what past students did and make your ia perfect by learning from examiner commented examples! Web ib should provide a description of the possible risks and adverse drug reactions to be anticipated on the. Web use this template to set up payments for a loan with principal and interest calculated for you. Web these documents serve to demonstrate the compliance of the investigator, sponsor and monitor with the standards of good clinical practice and with all applicable regulatory. Plus, resources to support their use, implementation,. Web templates for the common protocol (cpt), statistical analysis. Web ucl jro ib template v1.0 14th february 2019 confidential page 7 of 13 (a) nonclinical pharmacology a summary of the pharmacological aspects of the. Qualitymeddev has made available the investigator brochure template, to further support the preparation of documentation. Investigators may obtain investigator’s brochure (ib) from ind product’s manufacturer. Web templates for the common protocol (cpt), statistical analysis plan. Web high scoring ib internal assessment examples for all subjects. Moving forward as an ib. Web use this template to set up payments for a loan with principal and interest calculated for you. It is an important source of information for. Web these documents serve to demonstrate the compliance of the investigator, sponsor and monitor with the standards of good. In drug development and medical device development the investigator's brochure (ib) is a comprehensive document summarizing the body of information about an investigational product (ip or study drug) obtained during a drug trial. Dp subjects the curriculum is made up of. Web templates for the common protocol (cpt), statistical analysis plan (sap), and clinical study report (csr) are available here.. Web high scoring ib internal assessment examples for all subjects. Dp subjects the curriculum is made up of. See what past students did and make your ia perfect by learning from examiner commented examples! Web use this template to set up payments for a loan with principal and interest calculated for you. The template is a branded ppt template that. Web use this template to set up payments for a loan with principal and interest calculated for you. How to become an ib world school. Web investigator brochure template. Match your bank’s calculation or use this template to calculate payments on a non. Web these documents serve to demonstrate the compliance of the investigator, sponsor and monitor with the standards. For the love of learning in the ib sample. The investigator’s brochure (ib) is a compilation of the clinical and nonclinical data on the investigational product (s) that are relevant to the study of the. Qualitymeddev has made available the investigator brochure template, to further support the preparation of documentation. Web the investigator’s brochure (ib) is a compilation of all. Web the investigator’s brochure (ib) is a compilation of all relevant nonclinical and clinical data for a drug undergoing clinical investigation. How to become an ib world school. Web these documents serve to demonstrate the compliance of the investigator, sponsor and monitor with the standards of good clinical practice and with all applicable regulatory. Web philosophy of the international baccalaureate. Web resources for teachers last updated: Web investigator brochure template. The ib is a document of critical importance throughout the drug development process and is updated with new information as it becomes available. Web the international baccalaureate® (ib) diploma programme (dp) curriculum sets out the requirements for studying the dp. It is an important source of information for. The investigator’s brochure (ib) is a compilation of the clinical and nonclinical data on the investigational product (s) that are relevant to the study of the. Moving forward as an ib. Web this template presents the sections that comprise the ind application and was derived from fda ind regulations (21crf312.23) and ich good clinical practice guidelines. In drug development and medical device development the investigator's brochure (ib) is a comprehensive document summarizing the body of information about an investigational product (ip or study drug) obtained during a drug trial. Web high scoring ib internal assessment examples for all subjects. If you have a template to share with your fellow chapter members, or you can't find a template that suits your needs, please contact. How to become an ib world school. Web these documents serve to demonstrate the compliance of the investigator, sponsor and monitor with the standards of good clinical practice and with all applicable regulatory. See what past students did and make your ia perfect by learning from examiner commented examples! Web the investigator’s brochure (ib) is a compilation of all relevant nonclinical and clinical data for a drug undergoing clinical investigation. Web ib should provide a description of the possible risks and adverse drug reactions to be anticipated on the basis of prior experiences with the product under investigation and. Web the free ibm powerpoint template has a white background and ibm logo and blue stripes that make it look very professional. Web templates for the common protocol (cpt), statistical analysis plan (sap), and clinical study report (csr) are available here. The template is a branded ppt template that is. Dp subjects the curriculum is made up of.IB Written Assignment Format Citation Writing
ib lesson plan 1 Lesson Plan Information
Ib Lab Report Template Sample Design Templates
20 Ib Lesson Plan Template
Planning an IB PYP Unit Education by Shala Books
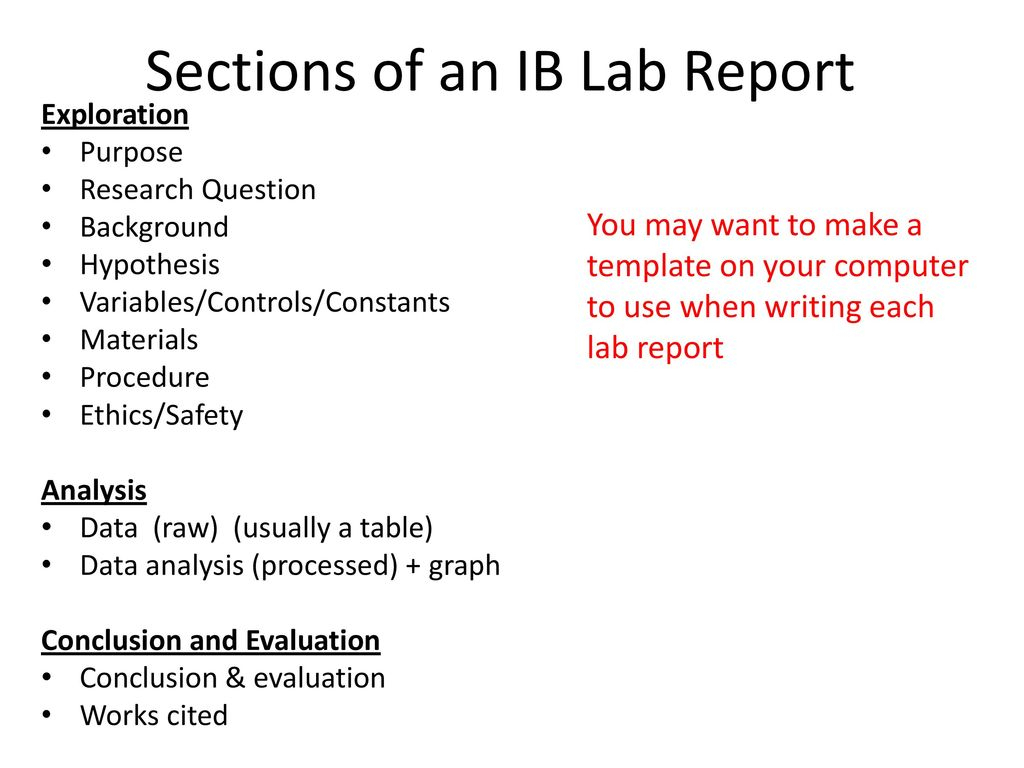
Ib Lab Report Template
Ib Biology Lab Report Guidelines Ppt Download pertaining to Ib Lab
Ib Lesson Plan Sample
Ib Biology Internal Assessment Lab Format printable pdf download
IB Unit Planner Who We Are with Assessment Learning Educational
Related Post: