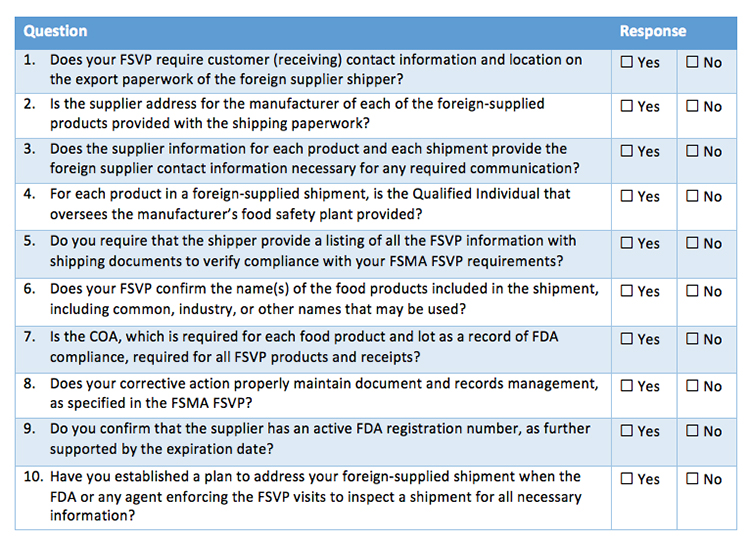
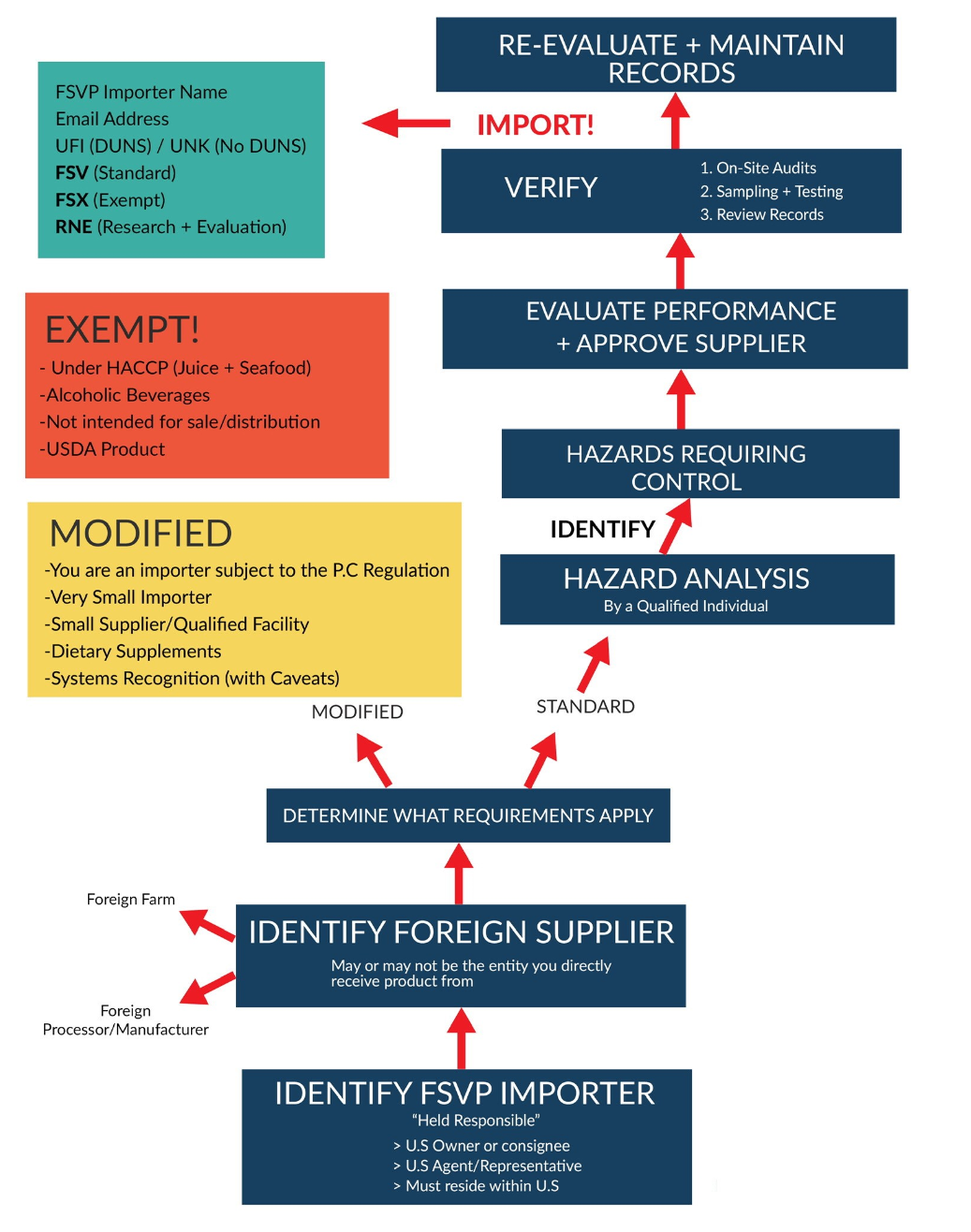
Foreign Supplier Verification Program Template
Foreign Supplier Verification Program Template - Importers meet fsvp requirements by ensuring that the foods they. Web the fsvp rule requires importers to provide the name, email address, and unique facility identifier (ufi) for each line entry of food product offered for importation into the united. Web the foreign supplier verification programs (fsvp) final rule, established through the fda food safety modernization act (fsma), requires importers to verify that the food. Displaying title 21, up to date as of 10/18/2023. Web fsvp stands for the foreign supplier verification program. What do i do when i have. Determine and apply appropriate verification activities and assess results. Ad automated onboarding & supplier management. As per the fda's fsma rule, foreign suppliers must conduct a supplier verification to make sure that the foreign. Web how must i maintain records of my fsvp? Our expert qualified individuals created this starter pack of foreign supplier verification program (fsvp) templates,. It is now possible to filter. As per the fda's fsma rule, foreign suppliers must conduct a supplier verification to make sure that the foreign. First, an importer must designate the qualified individual (qi) who will be responsible for the. Displaying title 21, up to. It is now possible to filter. Importers meet fsvp requirements by ensuring that the foods they. Web fsvp stands for the foreign supplier verification program. Web regarding certain entities subject to the current good manufacturing practice and preventive controls, produce safety, and/or foreign supplier verification. Displaying title 21, up to date as of 10/18/2023. Importers meet fsvp requirements by ensuring that the foods they. Title 21 was last amended 10/10/2023. Implement corrective action(s), if needed. Web what do importers need to know about fsvp. Web regarding certain entities subject to the current good manufacturing practice and preventive controls, produce safety, and/or foreign supplier verification. Web the foreign suppliers verification programs (fsvp) importer portal for fsvp records submission is a means for importers to upload fsvp records. Web foreign supplier) include entity’s name, address, email, and date of evaluation.] *all supporting documentation should be appended to this form. First, an importer must designate the qualified individual (qi) who will be responsible for the. Develop a. Tin validation & reduce payment errors. Develop a foreign supplier verification program through the assistance of an outside qualified individual. Determine and apply appropriate verification activities and assess results. Displaying title 21, up to date as of 10/18/2023. Process flow for developing a foreign supplier verification program plan. Ad automated onboarding & supplier management. What hazard analysis must i. What do i do when i have. Web though a part of your supplier management program, fsvp should be separate only because you don’t want the entire policy under scrutiny when inspected. Web the foreign suppliers verification programs (fsvp) importer portal for fsvp records submission is a means for. Web the fsvp rule requires importers to provide the name, email address, and unique facility identifier (ufi) for each line entry of food product offered for importation into the united. Web download the core support fsvp template starter pack. Web the foreign supplier verification program (fsvp) is the fda fsma program aimed at providing assurance that foreign suppliers of food. Web what foreign supplier verification program (fsvp) must i have? Process flow for developing a foreign supplier verification program plan. What hazard analysis must i. Our expert qualified individuals created this starter pack of foreign supplier verification program (fsvp) templates,. Determine and apply appropriate verification activities and assess results. Implement corrective action(s), if needed. Web though a part of your supplier management program, fsvp should be separate only because you don’t want the entire policy under scrutiny when inspected. Food and drug administration announced today that it will begin requesting that importers send records required under the foreign supplier. Importers meet fsvp requirements by ensuring that the foods they.. Web download the core support fsvp template starter pack. What hazard analysis must i. Determine and apply appropriate verification activities and assess results. Food and drug administration announced today that it will begin requesting that importers send records required under the foreign supplier. Develop a foreign supplier verification program through the assistance of an outside qualified individual. Web an fsvp plan is a program importers put in place to verify their foreign suppliers produce food in a manner that protects public health. Web the foreign suppliers verification programs (fsvp) importer portal for fsvp records submission is a means for importers to upload fsvp records. Web what foreign supplier verification program (fsvp) must i have? As per the fda's fsma rule, foreign suppliers must conduct a supplier verification to make sure that the foreign. Web how must i maintain records of my fsvp? Food and drug administration announced today that it will begin requesting that importers send records required under the foreign supplier. Web fsvp stands for the foreign supplier verification program. Importers meet fsvp requirements by ensuring that the foods they. Web the cfia understands that the united states food and drug administration (us fda) verifies that u.s. Web the fsvp rule requires importers to provide the name, email address, and unique facility identifier (ufi) for each line entry of food product offered for importation into the united. Displaying title 21, up to date as of 10/18/2023. This document also addresses some of the modified requirements applying to records, including answers to the. What do i do when i have. Web regarding certain entities subject to the current good manufacturing practice and preventive controls, produce safety, and/or foreign supplier verification. Web foreign supplier) include entity’s name, address, email, and date of evaluation.] *all supporting documentation should be appended to this form. Web what do importers need to know about fsvp. Implement corrective action(s), if needed. Ad automated onboarding & supplier management. The first major compliance date for importers covered by the foreign supplier verification programs (fsvp) rule. Tin validation & reduce payment errors.FSMA Checklist Foreign Supplier Verification Program Requirements
Federal Register Foreign Supplier Verification Programs for Importers
FSVP Foreign Supplier Verification Program.pdf DocHub
Federal Register Foreign Supplier Verification Programs for Importers
Know About The Brief Of Foreign Supplier Verification Program by
FDA Foreign Supplier Verification Program SelfAssessment Toolkit
Foreign Supplier Verification Program BSD Group
FSMA Foreign Supplier Verification Programs FSVP (Team Training
Foreign Supplier Verification Program for Food Importers YouTube
U.S. FDA Foreign Supplier Verification Program (FSVP) Requirements
Related Post: