Fda Software Validation Templates
Fda Software Validation Templates - Web fda software validation is a complex process. View our free patterns and checklist. Quality system regulation definitions 21 cfr. View our free template and checklist. View our loose template and. Trusted by leading pharma, biotech, and medical device companies globally. Web three (3) options to create a software validation protocol: Web click the thumbnail to access a free template. View our open template and checkout. The validation center™ library has the documents you. However, it does not list all of the activities and tasks that must, in all instances, be used. In this 2022 guidance we explaining what it is and methods for validate software. View our free template and checklist. Trusted by leading pharma, biotech, and medical device companies globally. Web three (3) options to create a software validation protocol: View our open template and checkout. In this 2022 guide we declare what computers is and how to validate software. View our free patterns and checklist. Review and approve the software system requirements. Web three (3) options to create a software validation protocol: Web fda software validation is a complex process. Web fda provides results from analytical testing of human and animal food and environmental samples without requiring the responsible party to submit a freedom of information act. Review and approve the software system requirements. Center for devices and radiological health center for biologics evaluation and research fda is issuing this draft guidance. Download software quality assurance sops and computer system validation templates. The validation center™ library has the documents you. Web fda software validation is a complex process. Quality system regulation definitions 21 cfr. Web three (3) options to create a software validation protocol: Web identify the necessary documentation for fda software validation. Web in this 2022 guides we explain what it is and how to cancel software. Web fda provides results from analytical testing of human and animal food and environmental samples without requiring the responsible party to submit a freedom of information act. You can create a software validation protocol, using a. Web fda software validation is a complex process. Set up an internal team for software validation. In this 2022 guide we explain what it is and how to validate software. You can create a software validation protocol, using a template. Review and approve the software system requirements. Web three (3) options to create a software validation protocol: Web software validation means confirmation by examination and provision of objective evidence that software specifications conform to user needs and intended uses, and. Web identify the necessary documentation for fda software validation. Web fda software validation is a complex process. Web verification means confirmation by examination and provision of objective. However, it does not list all of the activities and tasks that must, in all instances, be used. View our loose template and. Download software quality assurance sops and computer system validation templates. View our open template and checkout. In this 2022 guide we explain what it is and how to validate software. In this 2022 guide we declare what computers is and how to validate software. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in support of a pre. However, it does not list all of the activities and tasks that must, in all instances, be used. Web. Quality system regulation definitions 21 cfr. You can create a software validation protocol, using a template. Web this guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the validation of medical device software or the. View our free template and checklist. The validation center™ library has the documents you. Ad digitize and manage any validation, commissioning or qualification process. Quality system regulation definitions 21 cfr. You can create a software validation protocol, using a template. Web in this 2022 guides we explain what it is and how to cancel software. Web this guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the validation of medical device software or the. Web fda software validation is a complex process. Sop software validation sven piechottka. View our open template and checkout. Download software quality assurance sops and computer system validation templates. Web fda software validation is a complex process. In this 2022 guide we explain what it is and how to validate software. Web the fda currently advises that the level of validation should be parallel to the level of risk potential. However, it does not list all of the activities and tasks that must, in all instances, be used. Web identify the necessary documentation for fda software validation. Center for devices and radiological health center for biologics evaluation and research fda is issuing this draft guidance to. Outlines to scope of this check project and the mission for validate the software’s. Web fda software validation is a complex process. View our loose template and. The validation center™ library has the documents you. Web here exists a sampling fda software approval template:Software Validation Test Plan Template� Google Docs, Word, Apple
Software Validation Risk Assessment Template Master of
FDA Software Validation 2022 Guide, Checklist & Template (2022)
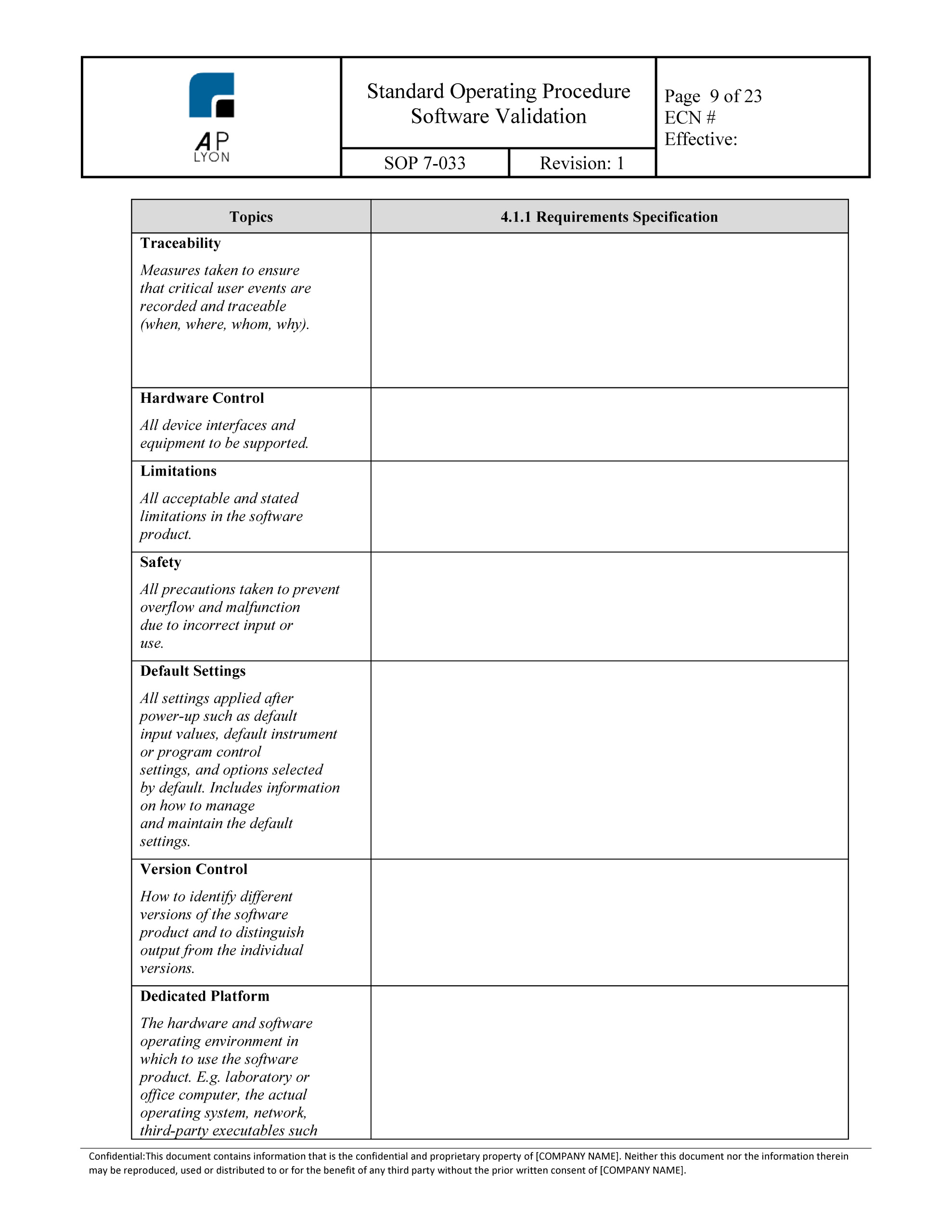
Software Validation Template
Template Word Master Software Validation Test Plan according to the
Software Validation Template
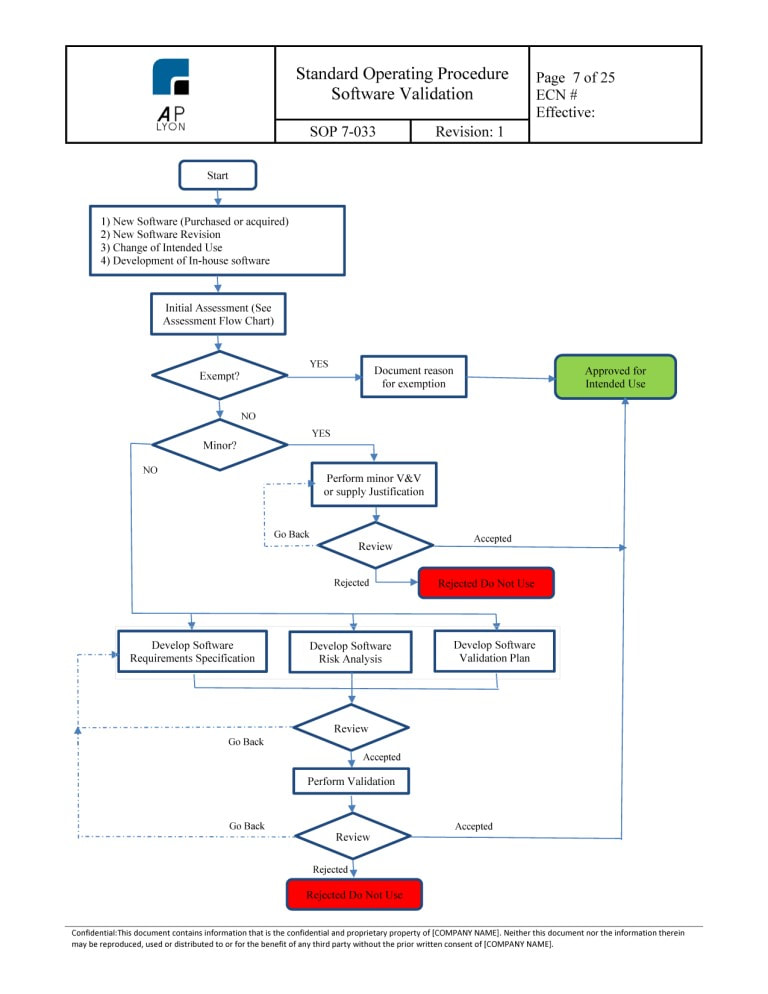
Software Validation Procedure
10+ Validation Plan Templates Sample Templates
FDA Software Validation 2022 Guide, Checklist & Template
FDA Guidance on Software Validation Terminology RegDesk
Related Post: