Fda Pre Submission Template
Fda Pre Submission Template - Web how to use the electronic submission template and resource (estar) pdf template. Send and track medical device premarket submissions online: Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and review information. Edit, sign and save fda 3601 form. Fda recommends the following about a sample submission: Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. Web clearly indicate what type of future submission (i.e. Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the fda. As of october 1, 2023, all 510 (k) submissions, unless exempted, must be submitted electronically using the finalized 510. Estar is an interactive pdf form that guides applicants. Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the fda. Web dockets management food and drug administration 5630 fishers lane, rm 1061 rockville, md 20852 all written comments should be identified with this document's. Estar is an interactive pdf form that guides applicants. Uslegalforms allows users to edit, sign, fill and. Web estar is the only available electronic submission template to prepare 510 (k) electronic submissions. Send and track medical device premarket submissions online: Web guidance for industry and food and drug administration staff. Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission.. Send and track medical device premarket submissions online: Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and review information. Web estar is the only available electronic submission template to prepare 510 (k) electronic submissions. Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the. Estar is an interactive pdf form that guides applicants. Web fda recommendations for sample submission. As of october 1, 2023, all 510 (k) submissions, unless exempted, must be submitted electronically using the finalized 510. Web guidance for industry and food and drug administration staff. However fda will not analyse any data or give a pass/fail to a result. Web without further ado, let’s jump into the first group. Additional regulatory tools and educational resources for. Web for medical device submissions: Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. As of october 1, 2023, all 510 (k) submissions, unless exempted,. Fda recommends the following about a sample submission: Web clearly indicate what type of future submission (i.e. Uslegalforms allows users to edit, sign, fill and share all type of documents online. Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. Web the. First, obtain a sample application number by emailing esub. Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and review information. Web estar is the only available electronic submission template to prepare 510 (k) electronic submissions. Experts preparing submissions for fda, health canada and european agencies Send and track medical device premarket submissions. Edit, sign and save fda 3601 form. Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and review information. As of october 1, 2023, all 510. Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. Edit, sign and save fda 3601 form. Uslegalforms allows users to edit, sign, fill and share all type of documents online. As of october 1, 2023, all 510 (k) submissions, unless exempted, must. Send and track medical device premarket submissions online: Estar is an interactive pdf form that guides applicants. Web estar is the only available electronic submission template to prepare 510 (k) electronic submissions. Fda recommends the following about a sample submission: Web how to use the electronic submission template and resource (estar) pdf template. Uslegalforms allows users to edit, sign, fill and share all type of documents online. As of october 1, 2023, all 510 (k) submissions, unless exempted, must be submitted electronically using the finalized 510. Ad providing the same outstanding quality of service across all electronic submissions. Web estar is the only available electronic submission template to prepare 510 (k) electronic submissions. Web dockets management food and drug administration 5630 fishers lane, rm 1061 rockville, md 20852 all written comments should be identified with this document's. Web fda recommendations for sample submission. Web how to use the electronic submission template and resource (estar) pdf template. Fda recommends the following about a sample submission: Web for medical device submissions: Additional regulatory tools and educational resources for. Experts preparing submissions for fda, health canada and european agencies Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and review information. Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the fda. Web clearly indicate what type of future submission (i.e. Web the presub is typically used to gain feedback on testing or protocols. First, obtain a sample application number by emailing esub. Estar is an interactive pdf form that guides applicants. Document issued on october 2, 2023. Web guidance for industry and food and drug administration staff. Document originally issued on september 22, 2022.A Quick & Easy Guide to FDA PreSubmissions
FDA 510(k) submission redacted
Fda Annual Report Cover Letter Template Online Cover Letter Library
Why the FDA PreSubmission is an Underutilized Tool
FDA Letter To 23andme PDF Federal Food Food And Drug Administration
FDA Draft Guidance on Electronic Submission Template for Medical Device
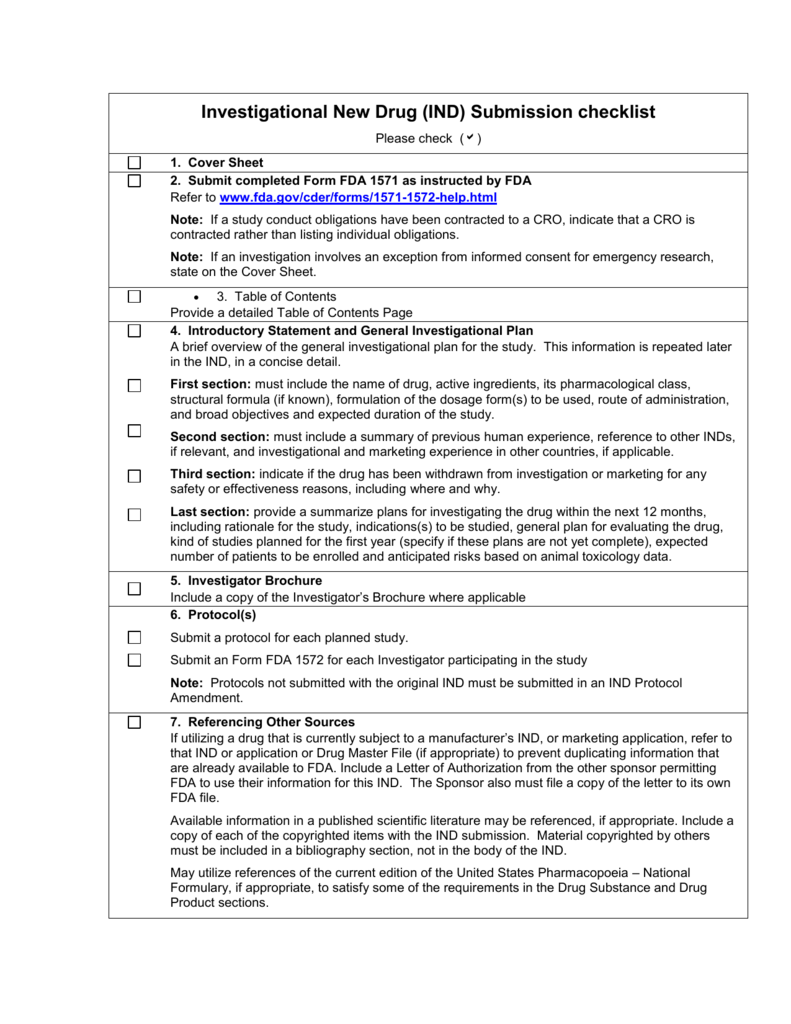
Investigational New Drug (IND) Submission checklist
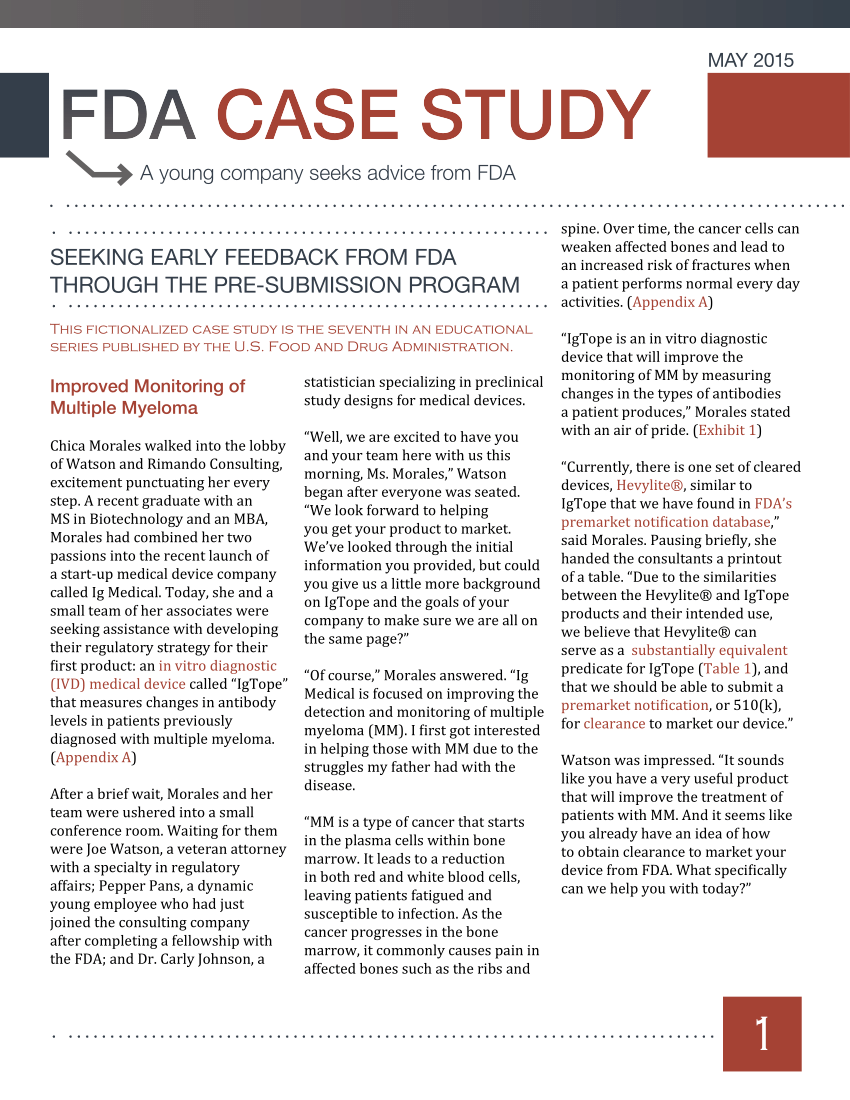
(PDF) Seeking early Feedback From FDA through the PreSubmission Program
FDA Pre Ind Meetings [PDF Document]
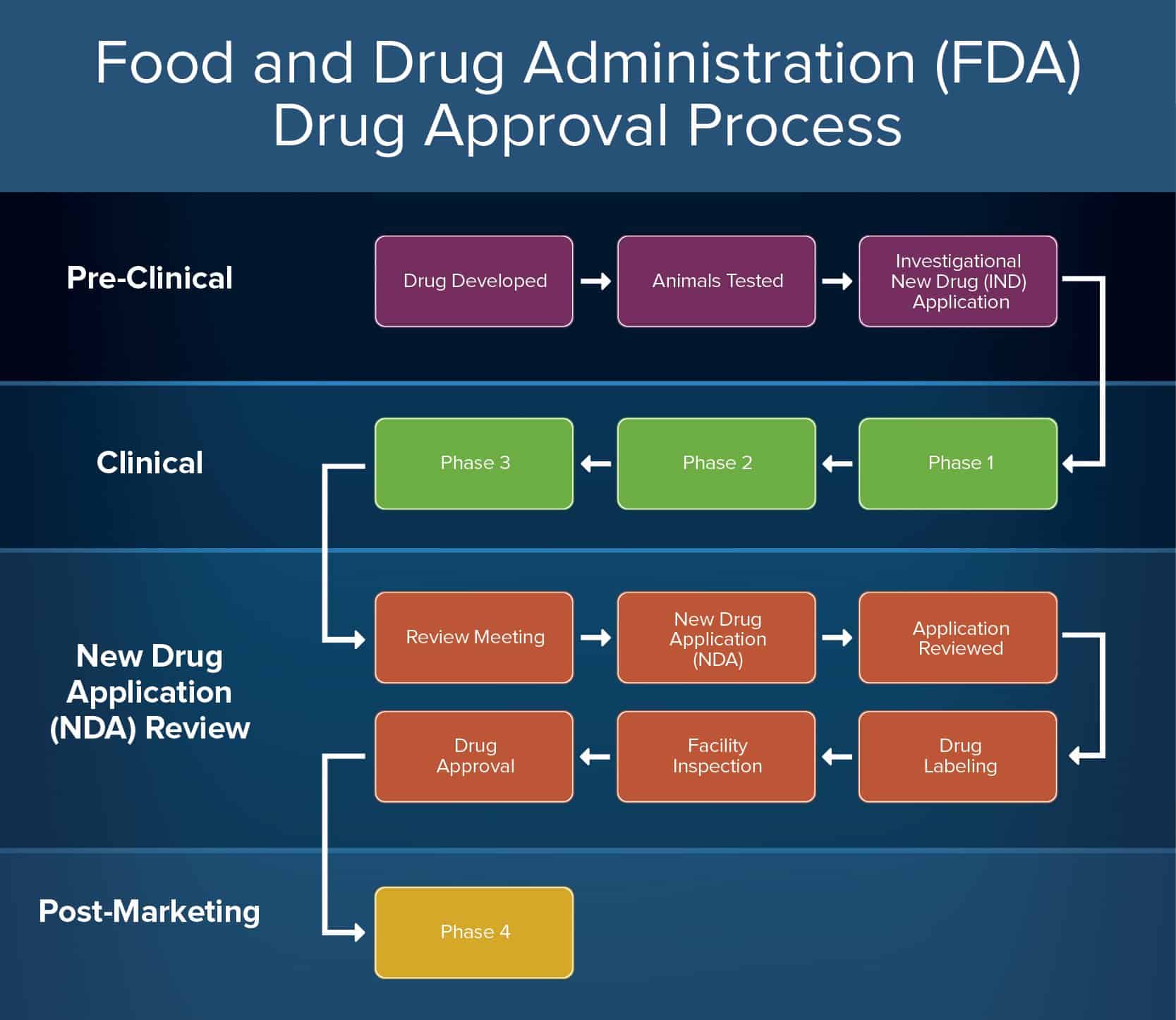
How to Create Approval Processes Smartsheet
Related Post:






![FDA Pre Ind Meetings [PDF Document]](https://static.documents.pub/img/1200x630/reader016/image/20180731/54f6fd2a4a7959430c8b4c0a.png?t=1615578926)