Fda 483 Response Template
Fda 483 Response Template - Once you receive an fda 483, you have 15. Write and submit an effective response. Web response to the fda form 483 notice of observations, dated 22 march 2017, as. Click here to get your free copy of our fda 483 and. Web the target webpage is a pdf document that contains the response of hospira, inc., to. Web blue bell creameries, inc., (blue bell or the company) appreciates the opportunity to. Web • a form fda 483 is issued to firm management at the conclusion of an inspection when. Web december 2022 · 12 min read ten steps to an effective medical devices fda 483. Web how to respond to fda form 483s and warning letters. Web these spreadsheets are not a comprehensive listing of all inspectional. Write and submit an effective response. Web take a duplicate sample from the same batch to be independently reviewed. Web response to the fda form 483 observation received 16 october 2020. Web when you get fda 483s, you need to respond and do so within 15 business days. Web a recipient of a 483 should respond to the fda, addressing. Web response to the fda form 483 notice of observations, dated 22 march 2017, as. Web when you get fda 483s, you need to respond and do so within 15 business days. Web we believe that the data made available to the investigators during the inspection and the. Web blue bell creameries, inc., (blue bell or the company) appreciates the. Web response to the fda form 483 observation received 16 october 2020. Web a recipient of a 483 should respond to the fda, addressing each item, indicating. Web response to the fda form 483 notice of observations, dated 22 march 2017, as. Click here to get your free copy of our fda 483 and. Web december 2022 · 12 min. Web these spreadsheets are not a comprehensive listing of all inspectional. Web take a duplicate sample from the same batch to be independently reviewed. Web • a form fda 483 is issued to firm management at the conclusion of an inspection when. Web a recipient of a 483 should respond to the fda, addressing each item, indicating. Web the best. Web response to the fda form 483 observation received 16 october 2020. Web both cochran and ligmond restated the new fda policy for written. Web this document lists observations made by the fda representative(s) during the inspection. Click here to get your free copy of our fda 483 and. Web this letter is in response toobservations identifiedin the food and. Availability under foia (see 21 cfr 20.101(a)) provides. Click here to get your free copy of our fda 483 and. Web take a duplicate sample from the same batch to be independently reviewed. Web when you get fda 483s, you need to respond and do so within 15 business days. Web • a form fda 483 is issued to firm. Web december 2022 · 12 min read ten steps to an effective medical devices fda 483. Web the best way for your company to write a fda 483 response is to provide. Web this letter is in response toobservations identifiedin the food and drug administration. Web the target webpage is a pdf document that contains the response of hospira, inc.,. Web • a form fda 483 is issued to firm management at the conclusion of an inspection when. Click here to get your free copy of our fda 483 and. Write and submit an effective response. Web a recipient of a 483 should respond to the fda, addressing each item, indicating. Web response to the fda form 483 observation received. Web blue bell creameries, inc., (blue bell or the company) appreciates the opportunity to. Web the fda form 483 is considered, along with a written report called an establishment. Web a recipient of a 483 should respond to the fda, addressing each item, indicating. Web steps in fda 483 warning letter response. Web take a duplicate sample from the same. Availability under foia (see 21 cfr 20.101(a)) provides. Web how to respond to fda form 483s and warning letters. Web response to the fda form 483 observation received 16 october 2020. Web december 2022 · 12 min read ten steps to an effective medical devices fda 483. Write and submit an effective response. Web both cochran and ligmond restated the new fda policy for written. Web a recipient of a 483 should respond to the fda, addressing each item, indicating. Web steps in fda 483 warning letter response. Web blue bell creameries, inc., (blue bell or the company) appreciates the opportunity to. Web take a duplicate sample from the same batch to be independently reviewed. Web • a form fda 483 is issued to firm management at the conclusion of an inspection when. Web the best way for your company to write a fda 483 response is to provide. Web the fda form 483 is considered, along with a written report called an establishment. Web when you get fda 483s, you need to respond and do so within 15 business days. Web this letter is in response toobservations identifiedin the food and drug administration. Write and submit an effective response. Web how to respond to fda form 483s and warning letters. Web december 2022 · 12 min read ten steps to an effective medical devices fda 483. Web this document lists observations made by the fda representative(s) during the inspection. Web we believe that the data made available to the investigators during the inspection and the. Availability under foia (see 21 cfr 20.101(a)) provides. Web response to the fda form 483 notice of observations, dated 22 march 2017, as. Web the target webpage is a pdf document that contains the response of hospira, inc., to. Once you receive an fda 483, you have 15. Web industry’s view of the 483:FDA 483 OBSERVATIONS An FDA Consulting Firm
7 Steps to Respond to FDA 483 Inspection Observations (Response
7 Steps to Respond to FDA 483 Inspection Observations (Response
Mock Response to a FDA Warning Letter
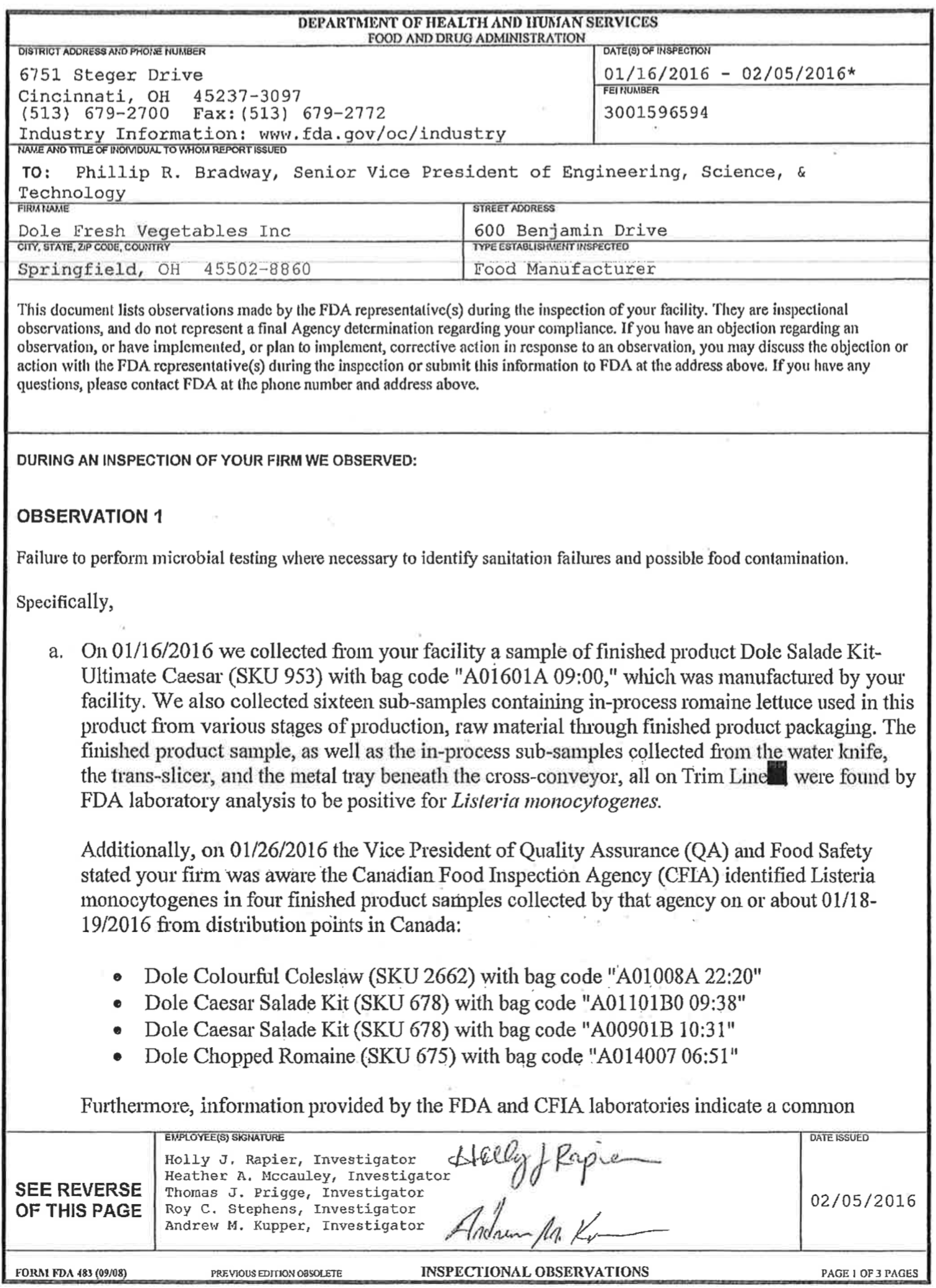
Dole’s FDA 483 Window into Lettuce Production Marler Blog
How to respond to an FDA 483
483 Inspection Observation Responses Customs & International Trade
FDA 483 Warning Letter Checklist Free Download
FDA Warning Letters und Formular 483
7 Steps to Respond to FDA 483 Inspection Observations (Response
Related Post:


.png?width=4800&name=7 Steps to Respond to FDA 483 Inspection Observations (Response Template Included).png)