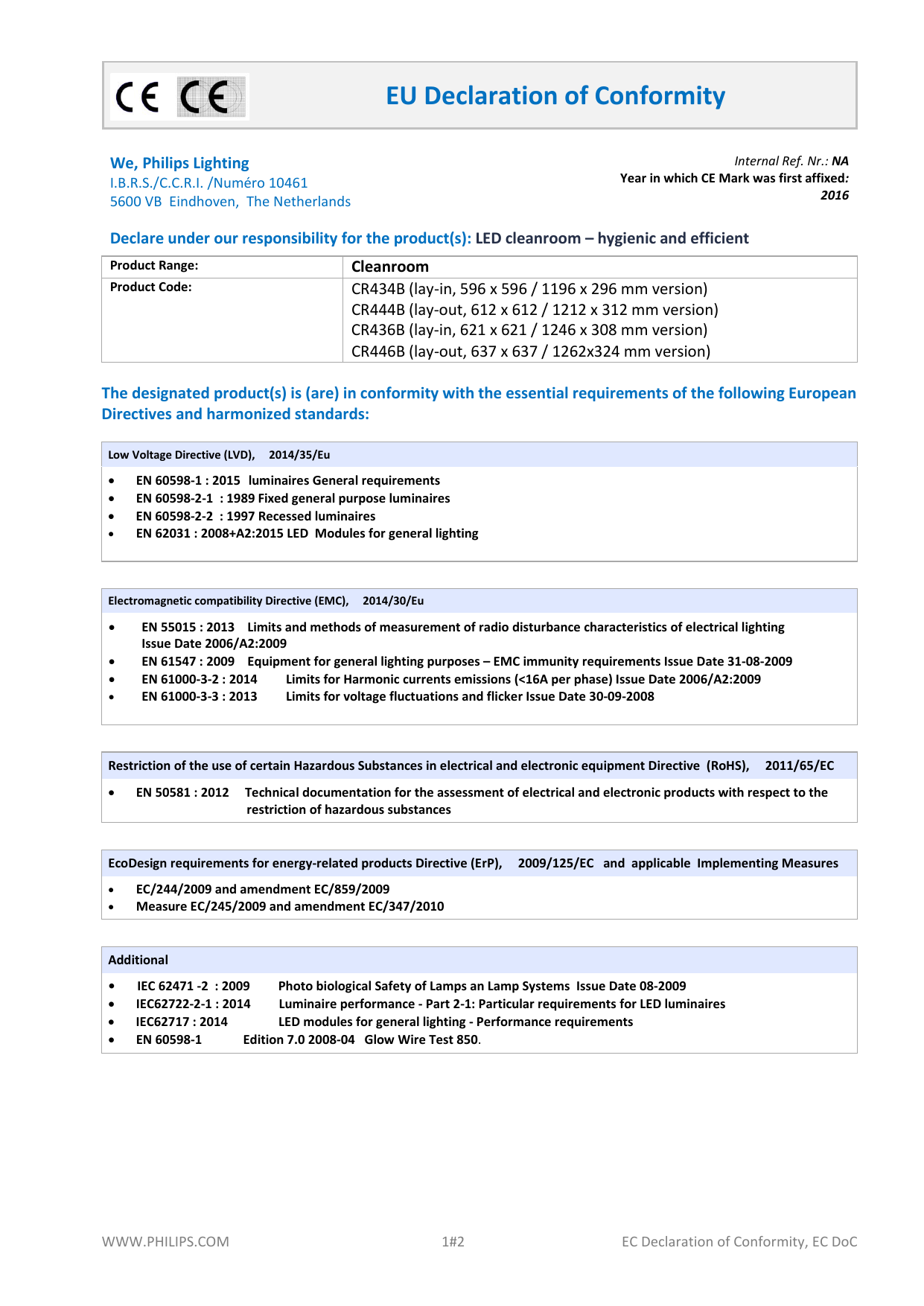
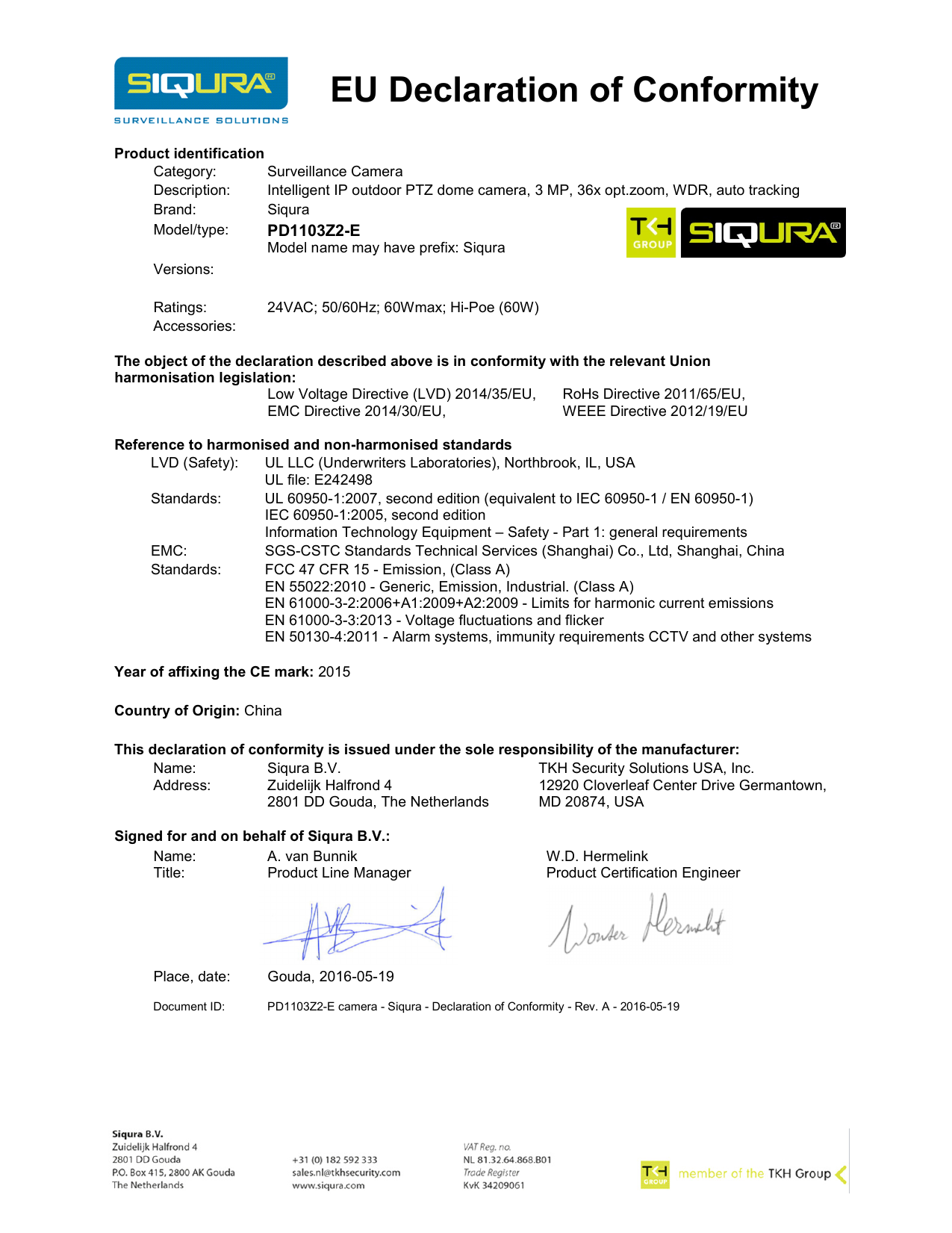
Eu Declaration Of Conformity Template
Eu Declaration Of Conformity Template - Ernest ken mayer \(emay\) subject: How a product complies with eu safety, health and environmental requirements, and how to place a ce marking on your product. Download native rendition (26.0) download pdf rendition (7.5380859375) ro. Of the regulation (eu) 2017/745. Web this declaration of conformity is issued under the sole responsibility of the manufacturer (or installer): Web the template the generic template of the (eu) declaration of conformity is given in annex iii of decision 768/2008/ec. Which information should be included in the technical documentation? Object of the declaration (identification of product allowing traceability. Web eu declaration of conformity cisco systems inc. Web declaration of conformity (doc) as a general requirement, the manufacturer of medical device or accessory in compliance with the applicable requirements of mdr 2017/745. & all its affiliates headquarter at: Web how to draft the eu statement of conformity. Web how can you prepare the technical documentation? Which information should be included in the technical documentation? Of the regulation (eu) 2017/745. Web print this page the government intends to introduce legislation to extend recognition of goods that meet eu requirements (including the ce marking ), indefinitely,. Web this declaration of conformity is issued under the sole responsibility of the manufacturer (or installer): Of the regulation (eu) 2017/745. 170 west tasman drive san jose, ca 95134 usa this declaration of conformity is.. Download native rendition (26.0) download pdf rendition (7.5380859375) ro. How to draft the eu declaration of. Web the eu declaration of conformity is the document in which the manufacturer states that the product fulfils the essential requirements of the applicable ce marking directives or. Web conformity assessment procedure: Web declaration of conformity (doc) as a general requirement, the manufacturer of. Web the template the generic template of the (eu) declaration of conformity is given in annex iii of decision 768/2008/ec. Of the regulation (eu) 2017/745. Web declaration of conformity (doc) as a general requirement, the manufacturer of medical device or accessory in compliance with the applicable requirements of mdr 2017/745. Additional information to be mentioned on. Web the eu declaration. Web a declaration of conformity (doc) is a contract written by a manufacturer or authorized person that confirms that the product placed on the market conforms to applicable eu. Of the regulation (eu) 2017/745. Web how to draft the eu statement of conformity. Object of the declaration (identification of product allowing traceability. How a product complies with eu safety, health. How a product complies with eu safety, health and environmental requirements, and how to place a ce marking on your product. Web print this page the government intends to introduce legislation to extend recognition of goods that meet eu requirements (including the ce marking ), indefinitely,. 170 west tasman drive san jose, ca 95134 usa this declaration of conformity is.. Web the eu declaration of conformity is the document in which the manufacturer states that the product fulfils the essential requirements of the applicable ce marking directives or. 170 west tasman drive san jose, ca 95134 usa this declaration of conformity is. Object of the declaration (identification of product allowing traceability. How to draft the eu declaration of. Web how. Web how can you prepare the technical documentation? Web as a manufacturer, you must carry out a risk analysis and ensure that your products comply with certain rules before placing them on the eu market. Of the regulation (eu) 2017/745. Web this declaration of conformity is issued under the sole responsibility of the manufacturer (or installer): Download native rendition (26.0). Web conformity assessment procedure: Web declaration of conformity (doc) as a general requirement, the manufacturer of medical device or accessory in compliance with the applicable requirements of mdr 2017/745. Web how to draft the eu statement of conformity. Of the regulation (eu) 2017/745. 170 west tasman drive san jose, ca 95134 usa this declaration of conformity is. Web as a manufacturer, you must carry out a risk analysis and ensure that your products comply with certain rules before placing them on the eu market. Ernest ken mayer \(emay\) subject: Web conformity assessment procedure: Which information should be included in the technical documentation? How to draft the eu declaration of. Conformation assessment procedure, notified bodies, technical standards, harmonised standards, risk assessment, ce marking. Web a declaration of conformity (doc) is a contract written by a manufacturer or authorized person that confirms that the product placed on the market conforms to applicable eu. Web how can you prepare the technical documentation? How a product complies with eu safety, health and environmental requirements, and how to place a ce marking on your product. How to draft the eu declaration of. & all its affiliates headquarter at: Additional information to be mentioned on. Ernest ken mayer \(emay\) subject: Which information should be included in the technical documentation? Web conformity assessment procedure: Web print this page the government intends to introduce legislation to extend recognition of goods that meet eu requirements (including the ce marking ), indefinitely,. Web declaration of conformity (doc) as a general requirement, the manufacturer of medical device or accessory in compliance with the applicable requirements of mdr 2017/745. Web how to draft the eu statement of conformity. Download native rendition (26.0) download pdf rendition (7.5380859375) ro. Web this declaration of conformity is issued under the sole responsibility of the manufacturer (or installer): Web a declaration of conformity (doc) is a legally binding document, in which the manufacturer asserts they have met the minimum requirements of the applicable. Object of the declaration (identification of product allowing traceability. 170 west tasman drive san jose, ca 95134 usa this declaration of conformity is. Of the regulation (eu) 2017/745. Web eu declaration of conformity cisco systems inc.EU Declaration of Conformity Siberia 150 Support
40 Free Certificate of Conformance Templates & Forms ᐅ TemplateLab
Declaration of conformity образец
EU Declaration of Conformity EastWest
Fillable Eu Declaration Of Conformity (Doc) printable pdf download
EN14960 EC Declaration of Conformity Rainbow Inflatables Ltd.
EU Declaration of Conformity Manualzz
How to write a Declaration of Conformity? (MDR and IVDR) Medical
EU Declaration of Conformity Manualzz
EU Declaration of Conformity Parallel Wireless
Related Post: