Ema Product Information Templates
Ema Product Information Templates - Web skip go main content. Web the european medicines agency's (ema) provides templates for product information for use by applicants and marketing authorisation holders of. Web the formatted table template is used by companies when they submit information on a medicine to the agency. How to prepare and review a summary of product characteristics; Web relevant european medicines agency’s templates: Web the product information templates are available on the european medicines agency (ema) website in all languages, including an annotated template in english. This page lists templates applicants may need for the preparation of their marketing authorisation. Ad download 100s of presentations, graphic assets, fonts, icons & more! Web changes will enhance presentation of information for patients and healthcare professionals. Product labels describe a medicinal product based on its. Web the european medicines agency's (ema) provides templates for product information for use by applicants and marketing authorisation holders of. Product labels describe a medicinal product based on its. On 10 june 2015, the european medicines agency published the revised human product information templates for medicinal products in. Web the formatted table template is used by companies when they submit. All the creative assets you need under one subscription! How to prepare and review a summary of product characteristics; Web the formatted table template is used by companies when they submit information on a medicine to the agency. Web ema/775752/2018 information management division statement of intent integration and replacement of formatted letter template with esubmission web ui functionality the. Start. Web the product information templates are available on the european medicines agency (ema) website in all languages, including an annotated template in english. How to prepare and review a summary of product characteristics; Web the formatted table template is used by companies when they submit information on a medicine to the agency. Web qrd human pi annotated template v10 1. Web an european medicinal agency's (ema) provides templates for product information for use by applicants and marketing authorizing holders starting veterinary. The following are those items of information. Web qrd human pi annotated template v10 1 version 10.3, 09/2022 annex i summary of product characteristics [note: Web the formatted table template is used by companies when they submit information on. Web skip to main content. Web the european pharmaceutical agency's (ema) provides templates for product information for uses by claimants or marketing authorisation holders of. All the creative assets you need under one subscription! How to prepare and review a summary of product characteristics; Ad we can help you achieve true agility by blending agile, product and technology. Web the product information templates are available on the european medicines agency (ema) website in all languages, including an annotated template in english. An implementation plan, also published today, outlines the. Web the formatted table template is used by companies when they submit information on a medicine to the agency. Web this template is used by companies to create the. Web the formatted table template is used by companies when they submit information on a medicine to the agency. Web an european medicinal agency's (ema) provides templates for product information for use by applicants and marketing authorizing holders starting veterinary. How to prepare and review a summary of product characteristics; Web ema/775752/2018 information management division statement of intent integration and. Web the formatted table template is used by companies when they submit information on a medicine to the agency. Web an european medicinal agency's (ema) provides templates for product information for use by applicants and marketing authorizing holders starting veterinary. Web the product information templates are available on the european medicines agency (ema) website in all languages, including an annotated. Web the product information templates are available on the european medicines agency (ema) website in all languages, including an annotated template in english. Web this template is used by companies to create the product information for the medicines they market in the eu. Ad download 100s of presentations, graphic assets, fonts, icons & more! Web the formatted table template is. Web ema/775752/2018 information management division statement of intent integration and replacement of formatted letter template with esubmission web ui functionality the. This page lists templates applicants may need for the preparation of their marketing authorisation. Web the european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for product information. Web the european. An implementation plan, also published today, outlines the. Web the european medicines agency (ema) has introduced a number of changes to the templates of the product information that accompany all medicines authorised for use. Web skip go main content. Web skip to main content. Web an european medicinal agency's (ema) provides templates for product information for use by applicants and marketing authorizing holders starting veterinary. It is intended to systematically structure and label the. Ad we can help you achieve true agility by blending agile, product and technology. Web the european pharmaceutical agency's (ema) provides templates for product information for uses by claimants or marketing authorisation holders of. Web the european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for product information. Web the european medicines agency's (ema) provides templates for product information for use by applicants and marketing authorisation holders of. Web changes will enhance presentation of information for patients and healthcare professionals. Start by assessing the information that needs to be included in your product. Web relevant european medicines agency’s templates: On 10 june 2015, the european medicines agency published the revised human product information templates for medicinal products in. Web ema/775752/2018 information management division statement of intent integration and replacement of formatted letter template with esubmission web ui functionality the. All the creative assets you need under one subscription! Web the product information templates are available on the european medicines agency (ema) website in all languages, including an annotated template in english. This page lists templates applicants may need for the preparation of their marketing authorisation. Web european medicines agency (ema) uses the summary of product characteristics (smpc) for product labels. Product labels describe a medicinal product based on its.FREE 49+ Information Sheet Samples in PDF MS Word
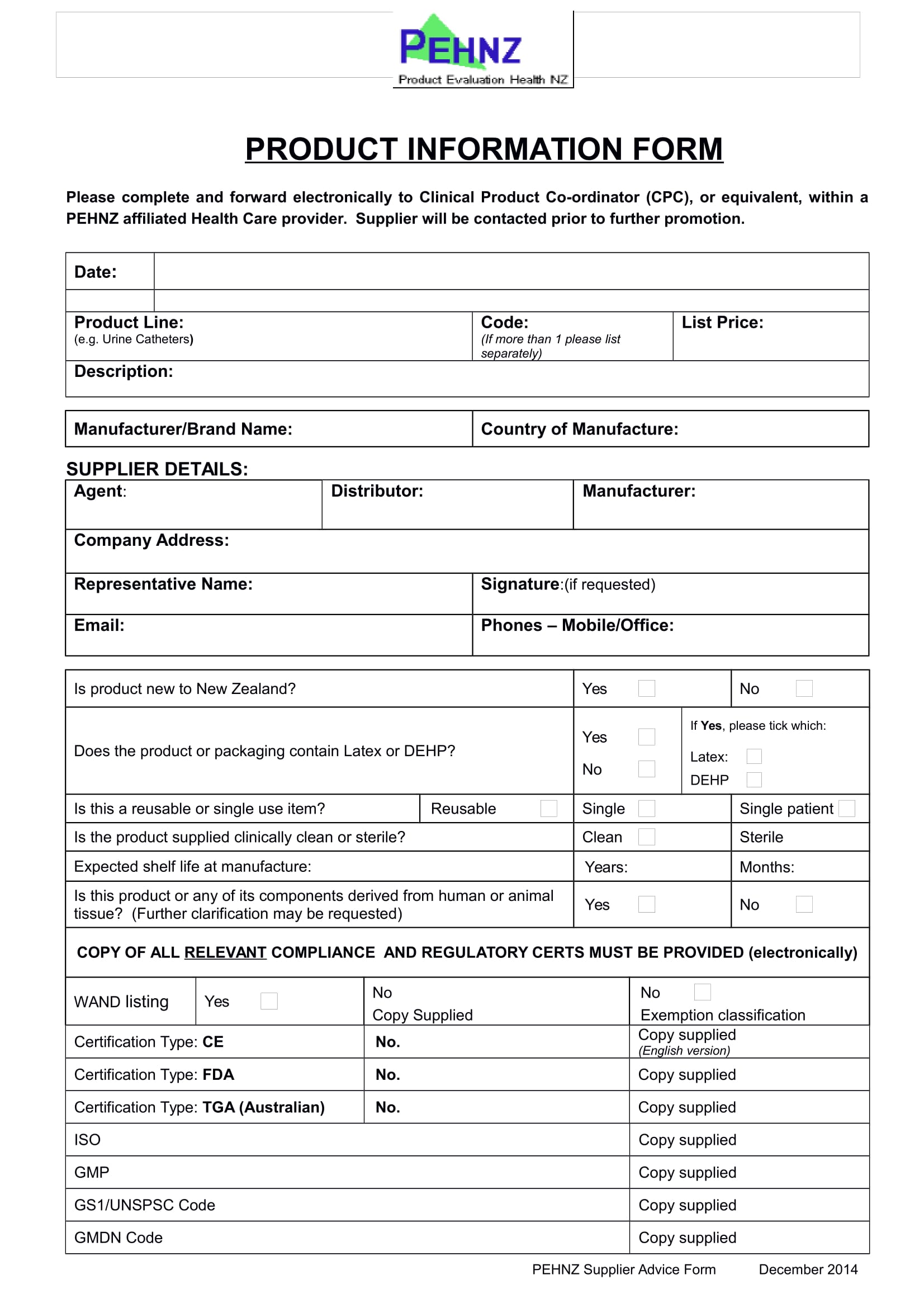
FREE 14+ Product Information Forms in MS Word PDF Excel
Top 10 OnePage New Product Fact Sheet Templates
FREE 14+ Product Information Forms in MS Word PDF Excel
Top 10 OnePage New Product Fact Sheet Templates
FREE 14+ Product Information Forms in MS Word PDF Excel
Veterinary Product Information Template Version 9 What did EMA change?
FREE 14+ Product Information Forms in MS Word PDF Excel
Product Data Sheet Templates Free Printable Templates
EMA GMP Guidelines For FP PDF Scientific Method Tablet (Pharmacy)
Related Post: