Data Management Plan Template Clinical Trial
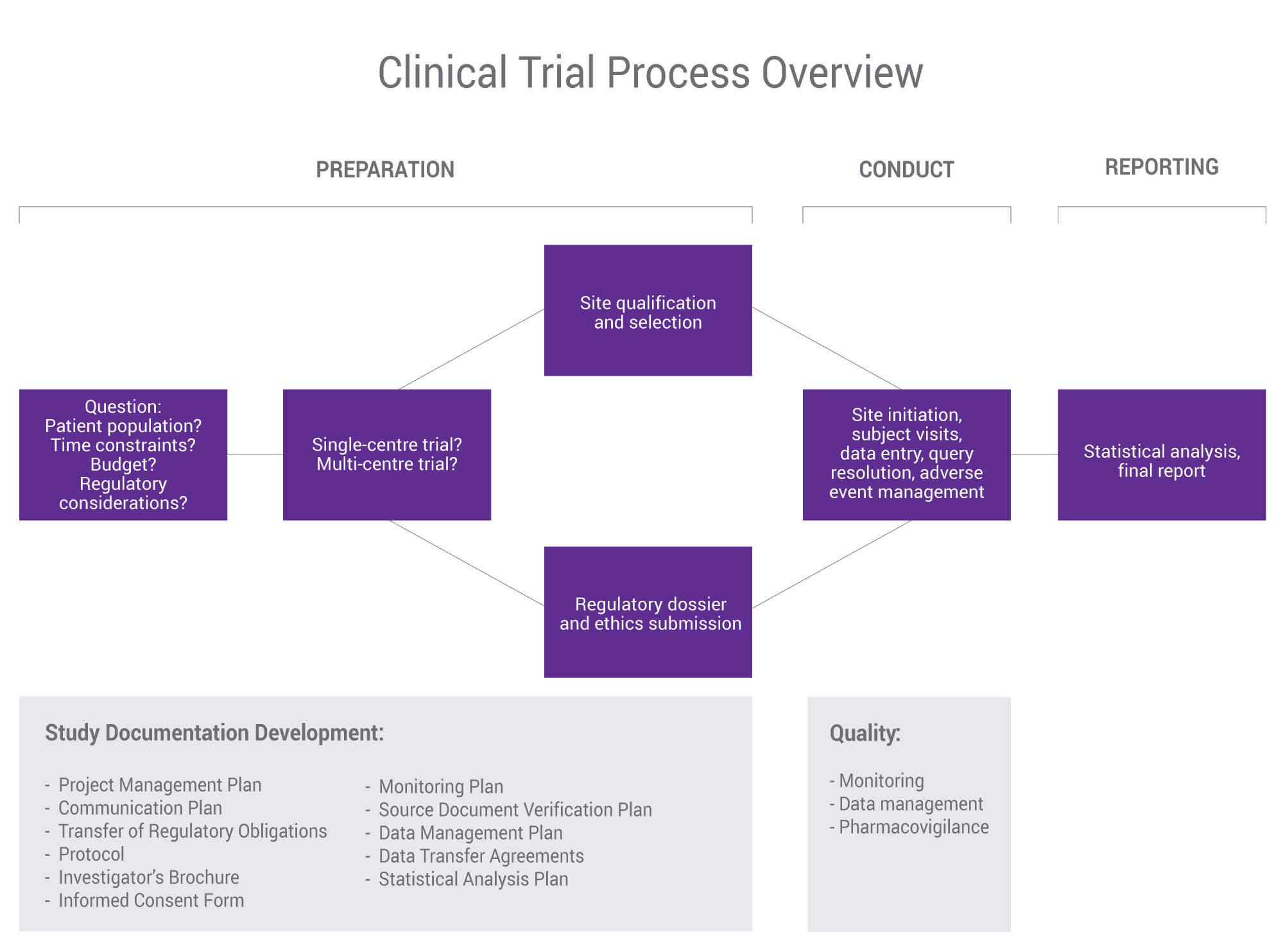
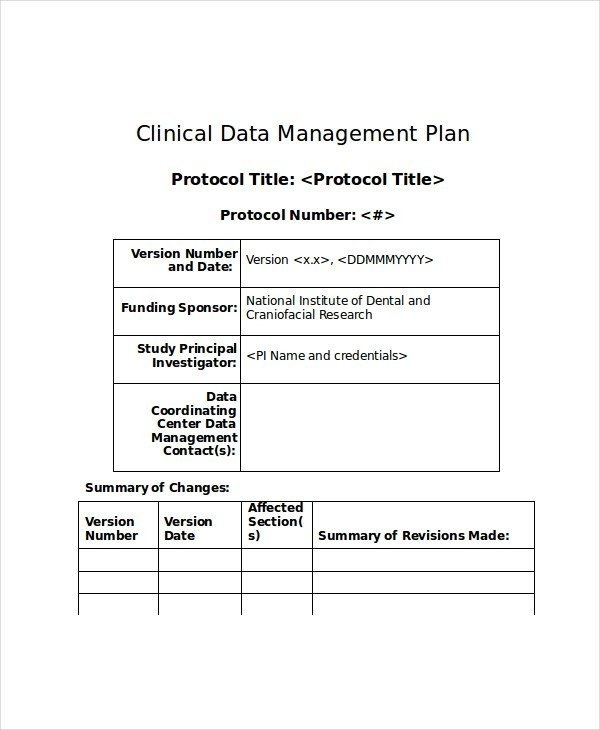
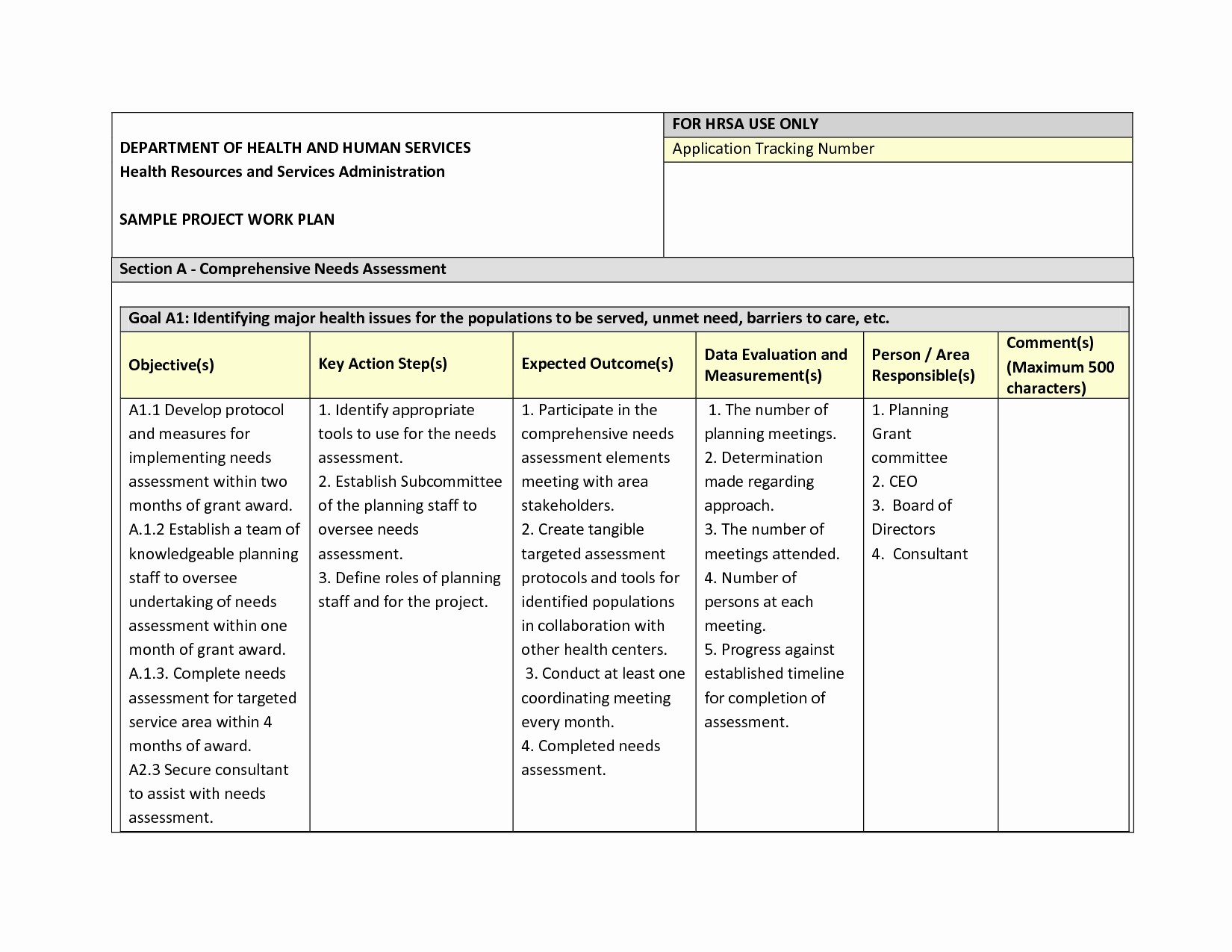
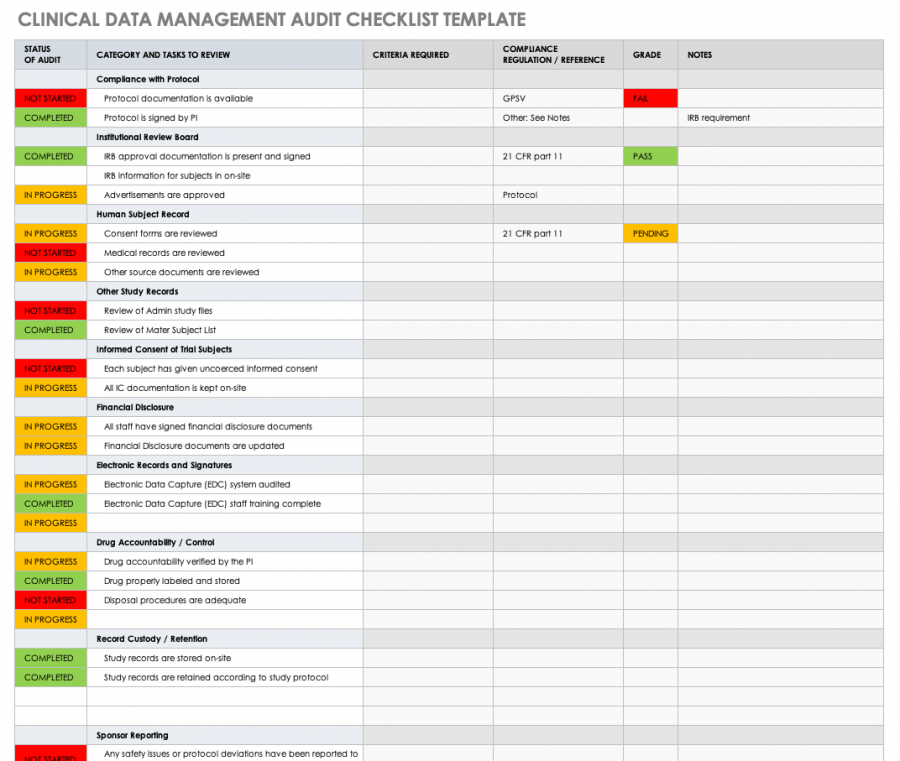
Data Management Plan Template Clinical Trial - Data & safety monitoring (dsmb, medical monitor, independent safety monitor) clinical site monitoring. Data handling study team agreement. Web clinical data management (cdm) is a critical phase in clinical research. Types and amount of scientific data expected to be generated in the project: Data management skills and experience Data & safety monitoring (csoc, medical monitor, independent safety monitor) clinical site monitoring. Web the dsmp should specify the following: Helps an investigator evaluate the data management system’s ability to capture and validate data that is collected on paper or electronically. Additionally, the sponsor may already have a data management plan template for clinical trials to follow. Potential risks and benefits for participating in the study. Dmptool is a fillable template that walks researchers through completing a plan that complies with funder requirements and has links to funder websites, help text for answering questions, and data. Web this template is created based on experience within the association of clinical data managers (acdm) and covers most aspects of data management (dm), you should however, always follow your. The design of the research data lifecycle should be strategized in the clinical data management plan (cdmp). Sample size and target population. If you are an nidcr grant applicant or awardee planning to conduct an interventional study, you may need the following. Provides a customizable template for studies using an electronic data capture (edc) system. Web this template is created. In 2013, phrma/efpia recognised the importance of data sharing and supported initiatives to enhance clinical trial data transparency and promote scientific advancements. Among the recommended items to include are: Data & safety monitoring (dsmb, medical monitor, independent safety monitor) clinical site monitoring. Data management skills and experience Safety and problem event reporting (aes, deviations, unanticipated problems, & pregnancies) quality management. Helps an investigator evaluate the data management system’s ability to capture and validate data that is collected on paper or electronically. The national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to ensure the safety of research participants. Web the dsmp should specify the following: Data management skills. In 2013, phrma/efpia recognised the importance of data sharing and supported initiatives to enhance clinical trial data transparency and promote scientific advancements. Web under the 2023 data management and sharing (dms) policy, nih expects researchers to maximize the appropriate sharing of scientific data, taking into account factors such as legal, ethical, or technical issues that may limit the extent of. Helps an investigator evaluate the data management system’s ability to capture and validate data that is collected on paper or electronically. The procedures content should include the methods used to assign and structure participant’s identifiers (id), the location of the id logs, the types of data collection instruments used, a description of. In the medical field, and the associated or. Helps an investigator evaluate the data management system’s ability to capture and validate data that is collected on paper or electronically. Clinical data management plan template. In the medical field, and the associated or affiliated sectors, reliability and security are not open to compromise. Many funding agencies, especially government sources, require a dmp as part of their application processes. Web. However, despite these commitments, recent. Presented by the faculty of medicine digital solutions data management team, faculty of medicine researchers. Sample size and target population. Additionally, the sponsor may already have a data management plan template for clinical trials to follow. Web a data management plan, or dmp, is a formal document that outlines how data will be handled during. Sample size and target population. Helps an investigator evaluate the data management system’s ability to capture and validate data that is collected on paper or electronically. Provides a customizable template for studies using an electronic data capture (edc) system. Web this data management plan template provides the required contents of a standard clinical trial data management plan, with space and. Web unit guarantees best practices in the field of clinical data management. Data & safety monitoring (dsmb, medical monitor, independent safety monitor) clinical site monitoring. The design of the research data lifecycle should be strategized in the clinical data management plan (cdmp). Sections may be edited or deleted as. Provides a customizable template for studies using an electronic data capture. This plan reviews work processes and project deliverables. The clinical data management procedures define the methods and dependent activities in which the clinical data is collected and managed. Web the clinical trial quality management plan (qmp) includes all the activities that verify that a trial is safe and meets its requirements. The procedures content should include the methods used to assign and structure participant’s identifiers (id), the location of the id logs, the types of data collection instruments used, a description of. Data handling study team agreement. Web clinical data management (cdm) is a critical phase in clinical research. Web this template is created based on experience within the association of clinical data managers (acdm) and covers most aspects of data management (dm), you should however, always follow your companies processes. Sample size and target population. The national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to ensure the safety of research participants. Sections may be edited or deleted as. Potential risks and benefits for participating in the study. Clinical data management plan template. Sections may be edited or deleted as. Web data management plan template. Data are physically stored in a [ oracle ver.12c/19c {for secutrial®} | mysql ver.7.x {for redcap tm} ] rdbms (relational database management system) using a dedicated cdms software (clinical database management system) [secutrial® | redcap tm]. Additionally, the sponsor may already have a data management plan template for clinical trials to follow. If you are an nidcr grant applicant or awardee planning to conduct an interventional study, you may need the following. Web data and safety monitoring plan (dsmp) template and guidelines (ms word, 37k) and dsmp checklist (ms word, 43k) were developed to assist investigators in preparation of a sound data and safety monitoring plan. These processes require deep knowledge and command of the underlying theories, principles, concepts and methods from the multiple disciplines informing clinical data management practice. Data & safety monitoring (dsmb, medical monitor, independent safety monitor) clinical site monitoring.All about Clinical Trial Data Management Smartsheet
Management Plans Al Trial Project Plan Example Template With Regard To
Free Clinical Trial Templates Smartsheet
Data Management Plan Medical Research Council
Data Management Plan 15+ Examples, Format, Pdf Examples
The Basics Of Clinical Trial Centralized Monitoring with regard to
Clinical Trial Project Management Plan Template
Data Management Plan 15+ Examples, Format, Pdf Examples
All about Clinical Trial Data Management Smartsheet
All about Clinical Trial Data Management Smartsheet
Related Post: