Consort Flow Diagram Template
Consort Flow Diagram Template - Web consort 2010 flow diagram assessed for eligibility (n= ) excluded (n= ) Web samples include bar graph, line graph, consort flowchart, path model, qualitative research figure, mixed methods research figure, illustration of experimental stimuli, and. It offers a standard way for authors to prepare reports of trial. Web in this project, a sas macro (sas® version 9.4) is developed to automate consort flow diagram generation from analysis data, adam adsl (subject level analysis dataset). Web a consort diagram presents the flow of subjects at each stage in a clinical trial. Mary and tim last modified by: Mary and tim created date: 50.9 kb (1 page) ( 4.5, 22 votes ) download or preview 1 pages of pdf version of sample template. Sample template for the consort diagram. Web consort flow diagram and checklist: Web consort 2010 flow diagram assessed for eligibility (n= ) excluded (n= ) Web the flow diagram included in each rct was assessed for completeness of reporting in relation to published criteria and the consort flow diagram template. Mary and tim last modified by: Diagrams of the flow of participants through a clinical trial are recommended in the consolidated standards. 50.9 kb (1 page) ( 4.5, 22 votes ) download or preview 1 pages of pdf version of sample template. Mary and tim last modified by: Web this project aims to: Web consort flow diagram and checklist: Manuscripts reporting the results of randomized trials must include the consort flow diagram showing the progress of patients. Web a consort diagram presents the flow of subjects at each stage in a clinical trial. Diagrams of the flow of participants through a clinical trial are recommended in the consolidated standards for reporting of trials (consort) statement, but it is. Web consort 2010 flow diagram assessed for eligibility (n= ) excluded (n= ) Sample template for the consort diagram.. Manuscripts reporting the results of randomized trials must include the consort flow diagram showing the progress of patients. Web samples include bar graph, line graph, consort flowchart, path model, qualitative research figure, mixed methods research figure, illustration of experimental stimuli, and. Web a consort diagram presents the flow of subjects at each stage in a clinical trial. Diagrams of the. Web hpb consort diagram template assessed for eligibility n = randomized n = excluded n = did not meet inclusion criteria n = refused to participate n = other reasons n =. Sample template for the consort diagram. Web the flow diagram can be accessed via the original published paper by following the pubmed links in the full bibliographic reference. Web a cross sectional review of all primary reports of randomized trials which included a consort flow diagram indexed in pubmed core clinical journals (2009). Manuscripts reporting the results of randomized trials must include the consort flow diagram showing the progress of patients. Consort 2010 checklist of information to include when reporting a randomized triala section and topic item no.. Consort 2010 checklist of information to include when reporting a randomized triala section and topic item no. Diagrams of the flow of participants through a clinical trial are recommended in the consolidated standards for reporting of trials (consort) statement, but it is. Web the flow diagram included in each rct was assessed for completeness of reporting in relation to published. Diagrams of the flow of participants through a clinical trial are recommended in the consolidated standards for reporting of trials (consort) statement, but it is. Web a consort diagram presents the flow of subjects at each stage in a clinical trial. Mary and tim last modified by: Mary and tim created date: Web hpb consort diagram template assessed for eligibility. It offers a standard way for authors to prepare reports of trial. Web in this project, a sas macro (sas® version 9.4) is developed to automate consort flow diagram generation from analysis data, adam adsl (subject level analysis dataset). Web the flow diagram can be accessed via the original published paper by following the pubmed links in the full bibliographic. Web consort flow diagram and checklist: Manuscripts reporting the results of randomized trials must include the consort flow diagram showing the progress of patients. Diagrams of the flow of participants through a clinical trial are recommended in the consolidated standards for reporting of trials (consort) statement, but it is. Web hpb consort diagram template assessed for eligibility n = randomized. Web a consort diagram presents the flow of subjects at each stage in a clinical trial. Web the flow diagram can be accessed via the original published paper by following the pubmed links in the full bibliographic reference section of this web page. 50.9 kb (1 page) ( 4.5, 22 votes ) download or preview 1 pages of pdf version of sample template. Diagrams of the flow of participants through a clinical trial are recommended in the consolidated standards for reporting of trials (consort) statement, but it is. It offers a standard way for authors to prepare reports of trial. Web consort flow diagram and checklist: Mary and tim created date: Mary and tim last modified by: Web in this project, a sas macro (sas® version 9.4) is developed to automate consort flow diagram generation from analysis data, adam adsl (subject level analysis dataset). Web consort 2010 flow diagram assessed for eligibility (n= ) excluded (n= ) Web the flow diagram included in each rct was assessed for completeness of reporting in relation to published criteria and the consort flow diagram template. Web samples include bar graph, line graph, consort flowchart, path model, qualitative research figure, mixed methods research figure, illustration of experimental stimuli, and. Sample template for the consort diagram. Consort 2010 checklist of information to include when reporting a randomized triala section and topic item no. Web hpb consort diagram template assessed for eligibility n = randomized n = excluded n = did not meet inclusion criteria n = refused to participate n = other reasons n =. Web this project aims to: Web a cross sectional review of all primary reports of randomized trials which included a consort flow diagram indexed in pubmed core clinical journals (2009). Manuscripts reporting the results of randomized trials must include the consort flow diagram showing the progress of patients.The CONSORT Flow Diagram Flow chart template, Process flow chart
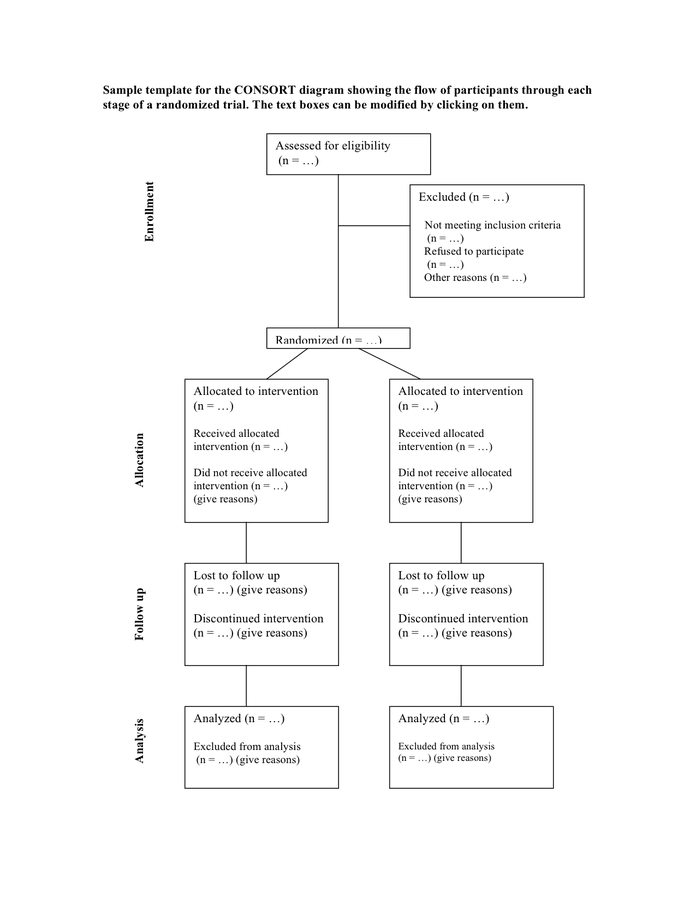
CONSORT diagram showing the flow of participants through each stage of
Sample template for the consort diagram in Word and Pdf formats
CONSORT 2010 flow diagram Download Scientific Diagram
CONSORT 2010 flow diagram. CONSORT flow diagram template courtesy of
The CONSORT flow diagram. Download Scientific Diagram
CONSORT 2010 flow diagram. Download Scientific Diagram
CONSORT flow diagram 16 . Download Scientific Diagram
Sample template for the consort diagram in Word and Pdf formats
Revised template of the CONSORT diagram showing the flow of
Related Post: