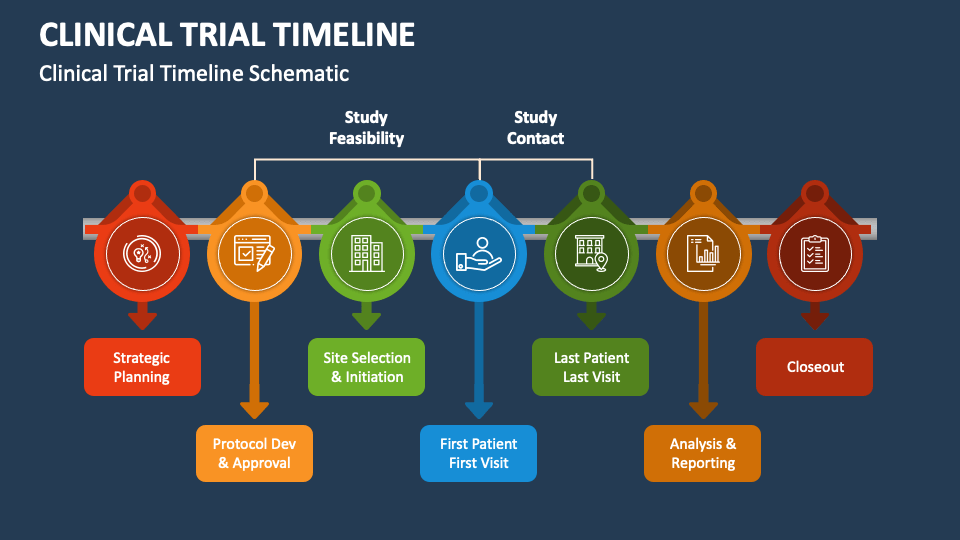
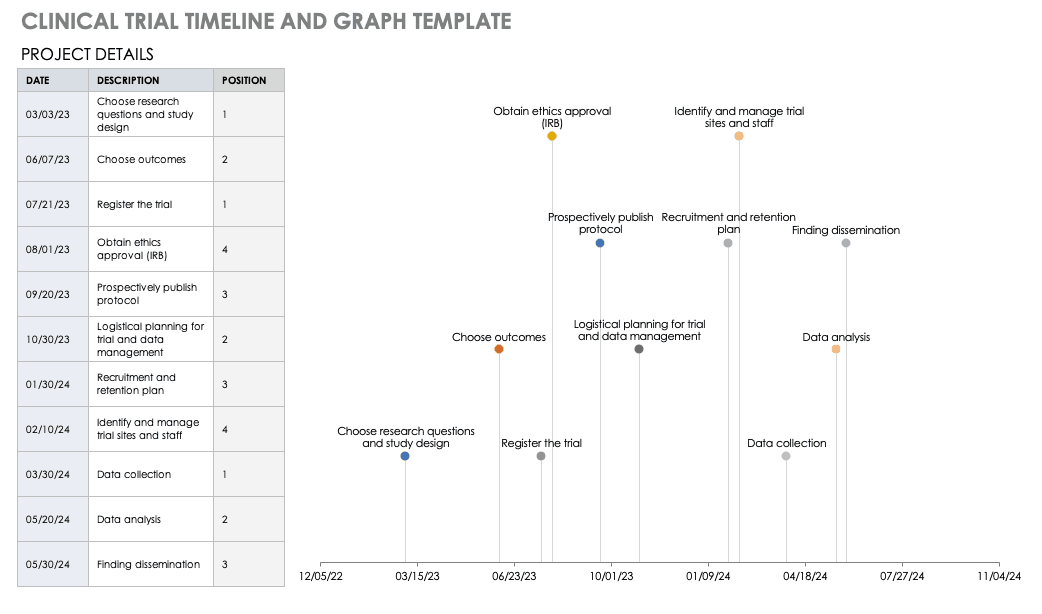
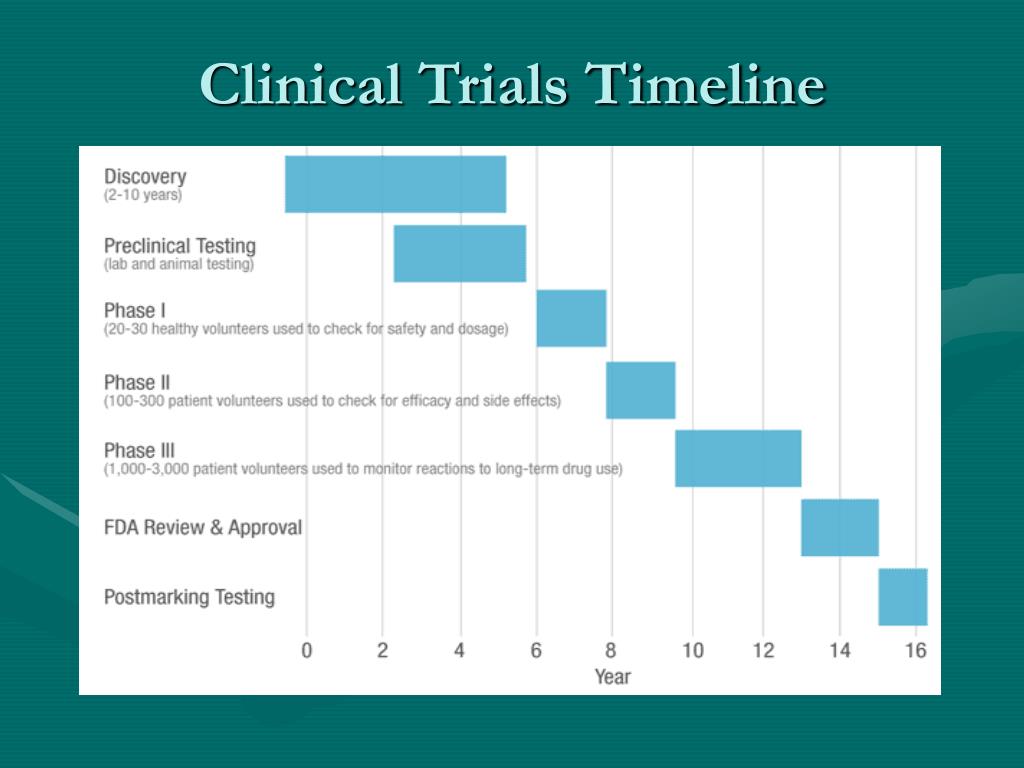
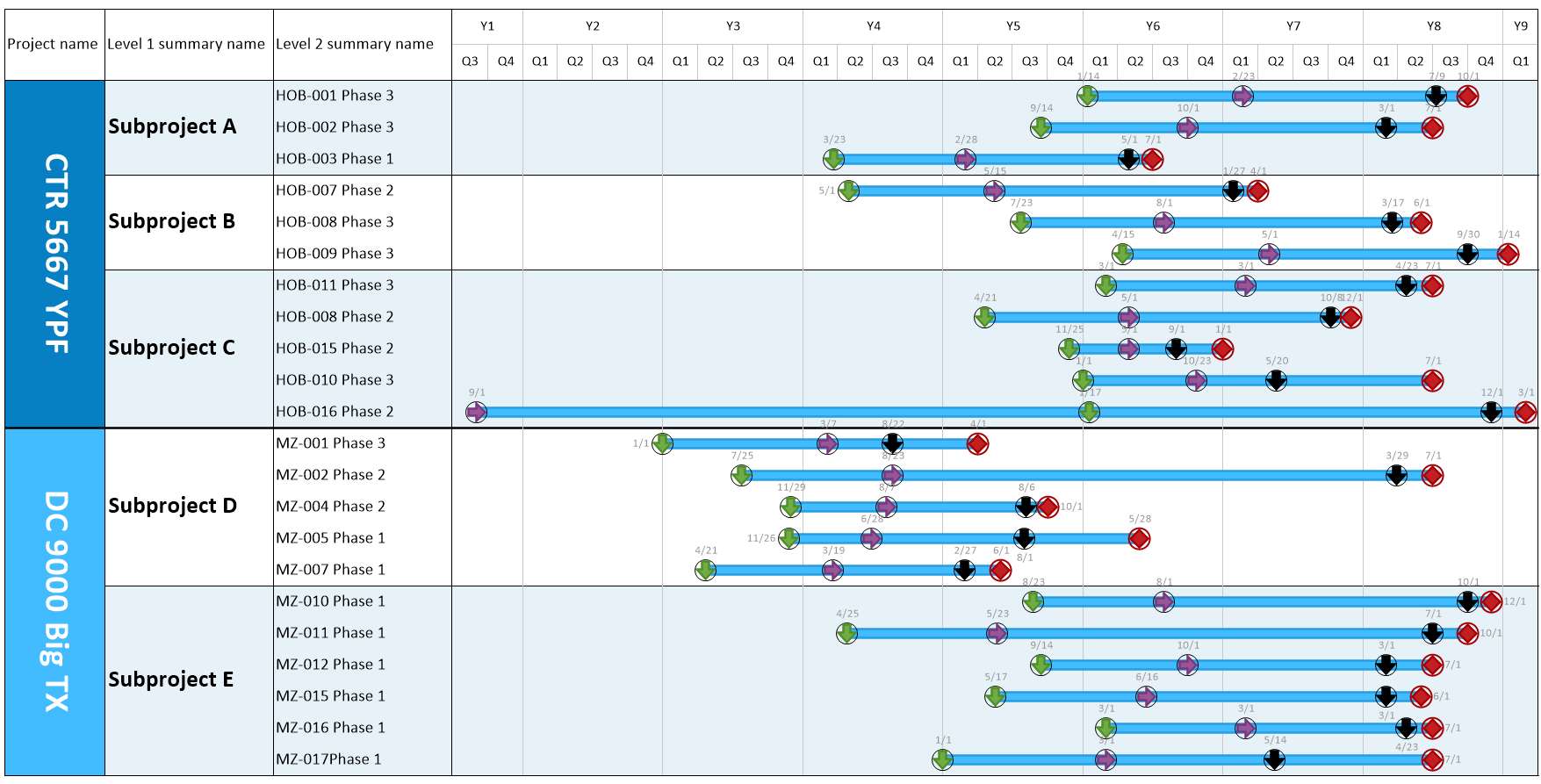
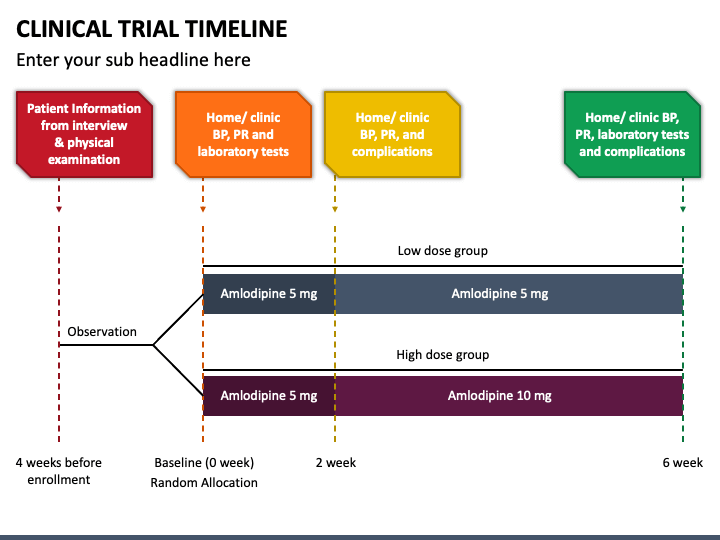
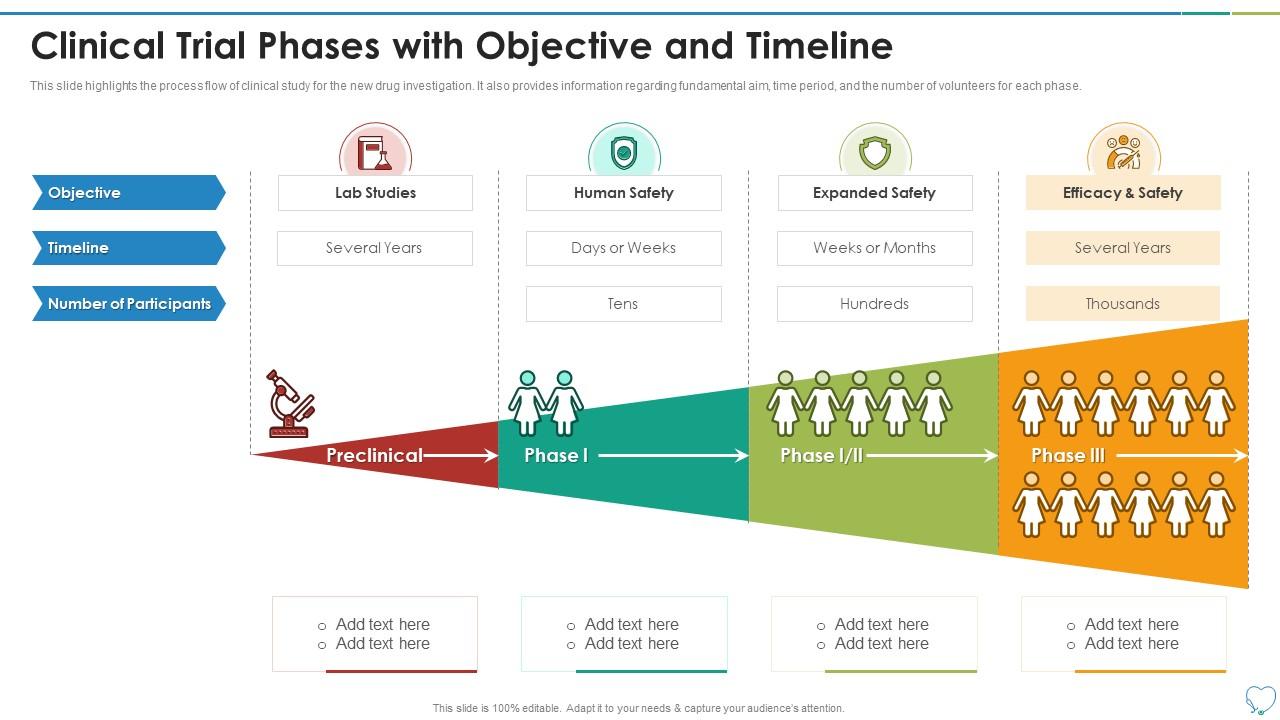
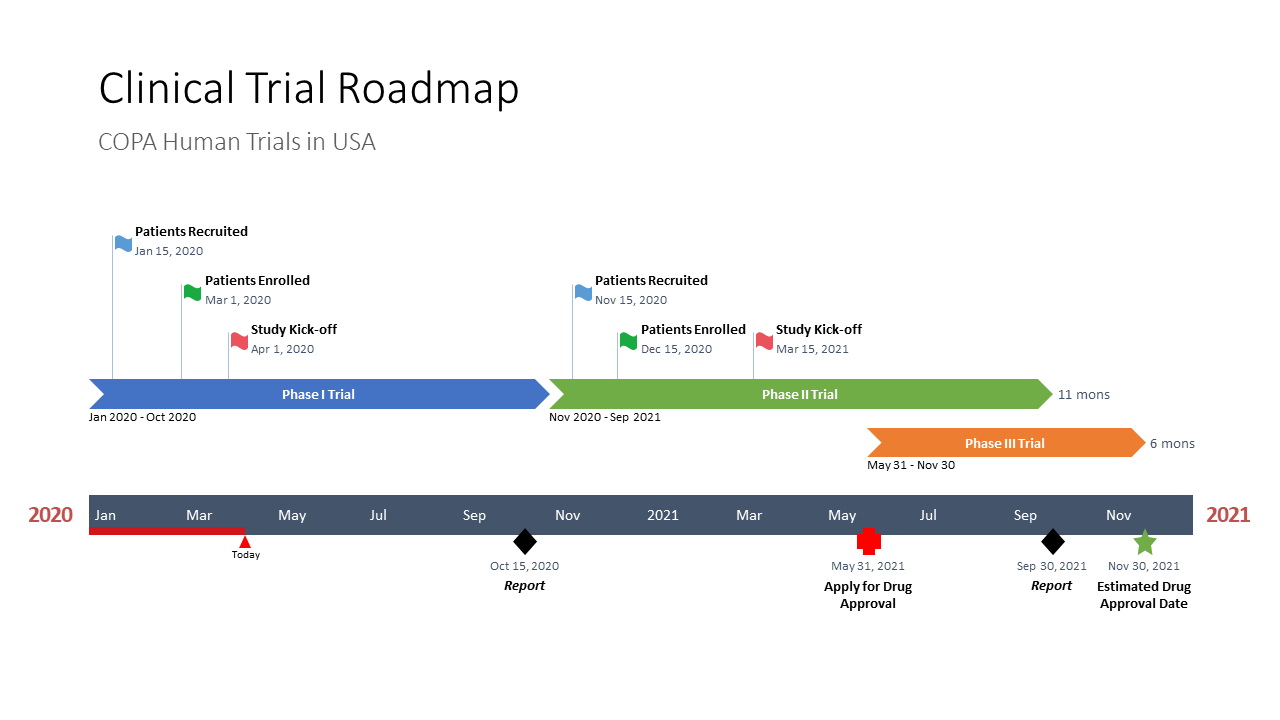
Clinical Trial Timeline Template
Clinical Trial Timeline Template - Welcome to global health trials' tools and templates library. This clinical milestone tracker was created in onepager express, timeline software for excel, using data exported from planisware ppm. The multicolored and unique graphics in this. Web a clinical trial management system (ctms) is a type of project management software specific to clinical research and clinical data management. Web clinical trial templates to start your clinical research. Number of treatments and data required. Program staff contacts pi to develop and submit milestones to nih via era commons. The type of drug or treatment being tested. This information can be very helpful to understanding the severity of the injuries and the impact that they have had on someone's life. When these timelines hyperlink to the actual treatment documents these timelines become an essential part of trial. Web the timeline can show the dates of the injuries, the types of treatment. Display of key milestone dates along the timeline. Phase 2 or 3 clinical trials that require investigational new drug applications (ind) or investigational device exemption (ide) applications The multicolored and unique graphics in this. This will help you stay on track and ensure that all necessary. Web clinical trial timeline template click here to create in smartsheet any articles, templates, or information provided by smartsheet on the website are for reference only. Program staff contacts pi to develop and submit milestones to nih via era commons. This clinical milestone tracker was created in onepager express, timeline software for excel, using data exported from planisware ppm. While. When these timelines hyperlink to the actual treatment documents these timelines become an essential part of trial. While we strive to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness,. The actual timeline of any clinical trial depends on multiple factors, some of which include: This. Welcome to global health trials' tools and templates library. The actual timeline of any clinical trial depends on multiple factors, some of which include: Please ensure that you read and adapt them carefully for your own setting, and that you reference global health trials and the global health network when you use. Phase 2 or 3 clinical trials that require. Web the timeline can show the dates of the injuries, the types of treatment. Display of key milestone dates along the timeline. Web specific symbols and colors for each type of milestone, for consistent reporting across trials. Web clinical trial templates to start your clinical research. The goals of the revision were to streamline and simplify the template by providing. Web the study management templates are adaptable and downloadable templates that can be used to organize and maintain research study documentation as you would include in a regulatory binder. Web clinical trial templates to start your clinical research. Guideline for budgeting for data and safety monitoring activities (ms word, 25k) aids investigators in budgeting for an independent dsmb or a. This information can be very helpful to understanding the severity of the injuries and the impact that they have had on someone's life. Web clinical trial timeline template click here to create in smartsheet any articles, templates, or information provided by smartsheet on the website are for reference only. This clinical trial milestone timeline was created using onepager, the leading. Phase 2 or 3 clinical trials that require investigational new drug applications (ind) or investigational device exemption (ide) applications This information can be very helpful to understanding the severity of the injuries and the impact that they have had on someone's life. This clinical milestone tracker was created in onepager express, timeline software for excel, using data exported from planisware. Web get our engrossing clinical trial timeline template, designed exclusively for microsoft powerpoint and google slides, to demonstrate the phases and key activities in the clinical trials. Web establish a timeline for your clinical trial, including key milestones and deadlines. Web a clinical trial management system (ctms) is a type of project management software specific to clinical research and clinical. Web clinical trial timeline template click here to create in smartsheet any articles, templates, or information provided by smartsheet on the website are for reference only. Web for behavioral and social clinical trials, consider using the adapted dsmp template (ms word, 62k). Display of key milestone dates along the timeline. Phase 2 or 3 clinical trials that require investigational new. Web a clinical trial management system (ctms) is a type of project management software specific to clinical research and clinical data management. Number of treatments and data required. The actual timeline of any clinical trial depends on multiple factors, some of which include: The multicolored and unique graphics in this. It allows for centralized planning, reporting, and tracking of all aspects of clinical trials, with the end goal of ensuring that the trials are efficient, compliant, and successful, whether across one. To learn more about how onepager can help you keep track of your clinical trials, get started today by downloading a free trial. Web specific symbols and colors for each type of milestone, for consistent reporting across trials. Web timeline for a typical milestone plan based on the assessed risk rating of the clinical trial. This clinical milestone tracker was created in onepager express, timeline software for excel, using data exported from planisware ppm. Phase 2 or 3 clinical trials that require investigational new drug applications (ind) or investigational device exemption (ide) applications When these timelines hyperlink to the actual treatment documents these timelines become an essential part of trial. Type and phase of trial. Web the study management templates are adaptable and downloadable templates that can be used to organize and maintain research study documentation as you would include in a regulatory binder. Display of key milestone dates along the timeline. This will help you stay on track and ensure that all necessary activities are completed within the desired timeframe. Program staff contacts pi to develop and submit milestones to nih via era commons. The goals of the revision were to streamline and simplify the template by providing clear and concise instructions to the authors, eliminating duplicative sections and information. The type of drug or treatment being tested. Web for behavioral and social clinical trials, consider using the adapted dsmp template (ms word, 62k). The templates below have been shared by other groups, and are free to use and adapt for your research studies.Clinical Trial Timeline PowerPoint and Google Slides Template PPT Slides
Clinical Trial RoadmapTablesDiagram
Clinical Trial Infographics for Google Slides and PowerPoint
Free Clinical Trial Templates Smartsheet
PPT GCP 101 or Why we do What we do the Way we do it Elaine Dempsey
OnePager Clinical Trial Milestone Timeline
Clinical Trial Timeline PowerPoint Template PPT Slides
Clinical Trial Phases Objective And Timeline Presentation Graphics
3 Best Free Templates for Pharmaceutical Timelines Project management
Pharmaceutical Trial Free Timeline Template Timeline in powerpoint
Related Post: