Clinical Trial Protocol Template
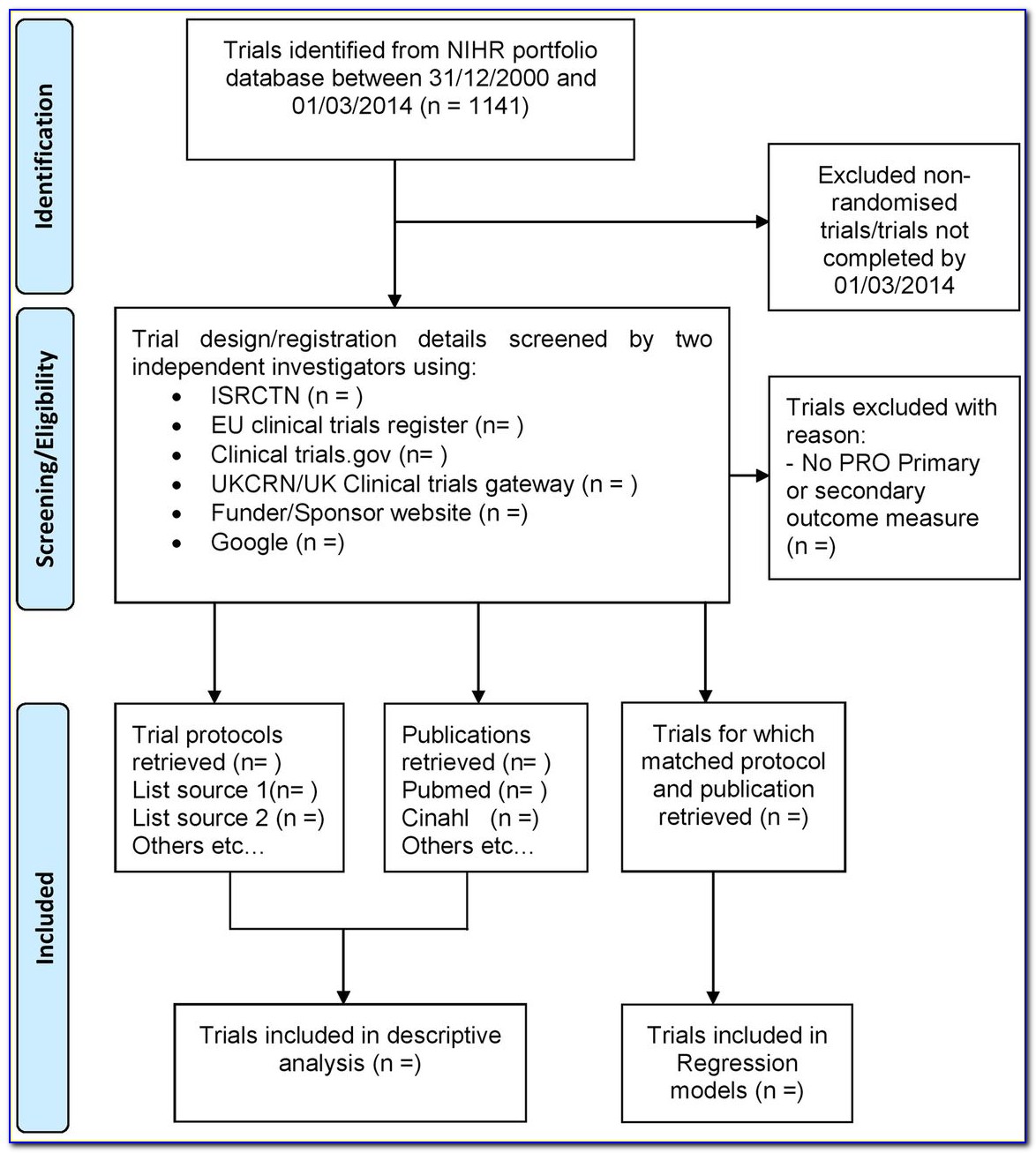
Clinical Trial Protocol Template - Web protocol templates for clinical trials. Web guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice and human subject protection. Web suggested templates for phase 1 and 2 clinical trials generic protocol documents and instructions for ctep studies instructions for submitting protocol. This template is intended to be used for clinical trials. Clinical research proposal form in pdf. Web basic clinical research proposal template. Web this trial protocol has been provided by the authors to give readers additional information about their work. In 2017, the allergy patch failed to meet its primary endpoint in the phase iii pepites clinical. Web there are two templates to be used for interventional research: Clinical trial program research proposal template. In 2017, the allergy patch failed to meet its primary endpoint in the phase iii pepites clinical. Web of nih’s more than $17b investment, clinical trials (~$7b) reflect the point at which the public is most directly engaged in nih’s clinical research activities, either as dedicated. Walsh ee, frenck rw jr, falsey ar, et al. Web the sample will consist. Web this study protocol was prepared in accordance with the standard protocol items for randomized trials (spirit) statement (chan et al., 2015). Web this clinical trial agreement template makes that process easier by streamlining the process of creating a contract between a sponsor and institution. Web the viaskin patch’s approval process has been a tumultuous one. Clinical trials are intended. Web this template is intended for interventional clinical trials of drugs, vaccines, and drug/device combinations intended to be registered as drugs. Web the viaskin patch’s approval process has been a tumultuous one. Web protocol templates for clinical trials. Web eim/allegra information added to protocol templates for research utilizing penn state health patient data, submissions using eim (enterprise information management) must.. Clinical trials are intended in their broadest sense and. Walsh ee, frenck rw jr, falsey ar, et al. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types. Web a clinical trial agreement is a legal document that serves the purpose of managing the relationship between the institution that is. Web a clinical trial agreement is a legal document that serves the purpose of managing the relationship between the institution that is conducting the clinical study in a region in. Clinical research proposal form in pdf. 100 patients (bipolar and major depressive disorder) who are treated with either ect (n = 50) or tms (n = 50) and. Web guidance. Clinical trials are intended in their broadest sense and. Web a clinical trial agreement is a legal document that serves the purpose of managing the relationship between the institution that is conducting the clinical study in a region in. 100 patients (bipolar and major depressive disorder) who are treated with either ect (n = 50) or tms (n = 50). Web intervention study template (clinical trials): Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a food. Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with. Ad put the. Walsh ee, frenck rw jr, falsey ar, et al. Web this study protocol was prepared in accordance with the standard protocol items for randomized trials (spirit) statement (chan et al., 2015). Web the viaskin patch’s approval process has been a tumultuous one. This template is intended to be used for clinical trials. Web this clinical trial agreement template makes that. Web phase 1 clinical trial protocol template. Ad put the experience of our mobile research nurses to work for your trial. Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with. Web a clinical trial agreement is a legal document that serves the purpose of managing the relationship between the institution. This template is intended to be used for clinical trials. Web phase 1 clinical trial protocol template. Web this study protocol was prepared in accordance with the standard protocol items for randomized trials (spirit) statement (chan et al., 2015). Web a clinical trial agreement is a legal document that serves the purpose of managing the relationship between the institution that. Web suggested templates for phase 1 and 2 clinical trials generic protocol documents and instructions for ctep studies instructions for submitting protocol. Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a food. Web this study protocol was prepared in accordance with the standard protocol items for randomized trials (spirit) statement (chan et al., 2015). Clinical trial program research proposal template. Web this trial protocol has been provided by the authors to give readers additional information about their work. Web a clinical trial agreement is a legal document that serves the purpose of managing the relationship between the institution that is conducting the clinical study in a region in. Web phase 1 clinical trial protocol template. Web the viaskin patch’s approval process has been a tumultuous one. Walsh ee, frenck rw jr, falsey ar, et al. Learn why we're a preferred mobile research provider for decentralized clinical trials. Web this template is intended for interventional clinical trials of drugs, vaccines, and drug/device combinations intended to be registered as drugs. In 2017, the allergy patch failed to meet its primary endpoint in the phase iii pepites clinical. Web eim/allegra information added to protocol templates for research utilizing penn state health patient data, submissions using eim (enterprise information management) must. Clinical research proposal form in pdf. Web a master protocol document (‘mpd’ or ‘protocol’) is a repository for accumulated ideas, information, literature review, and references. This template is intended to be used for clinical trials. Web intervention study template (clinical trials): Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types. Web of nih’s more than $17b investment, clinical trials (~$7b) reflect the point at which the public is most directly engaged in nih’s clinical research activities, either as dedicated.Standard Protocol Template NIHR Clinical Research Network
Free Clinical Trial Templates Smartsheet With Clinical Trial Report
Sample Research Protocol Clinical Trial Risk
Nih Fda Clinical Trial Protocol Template
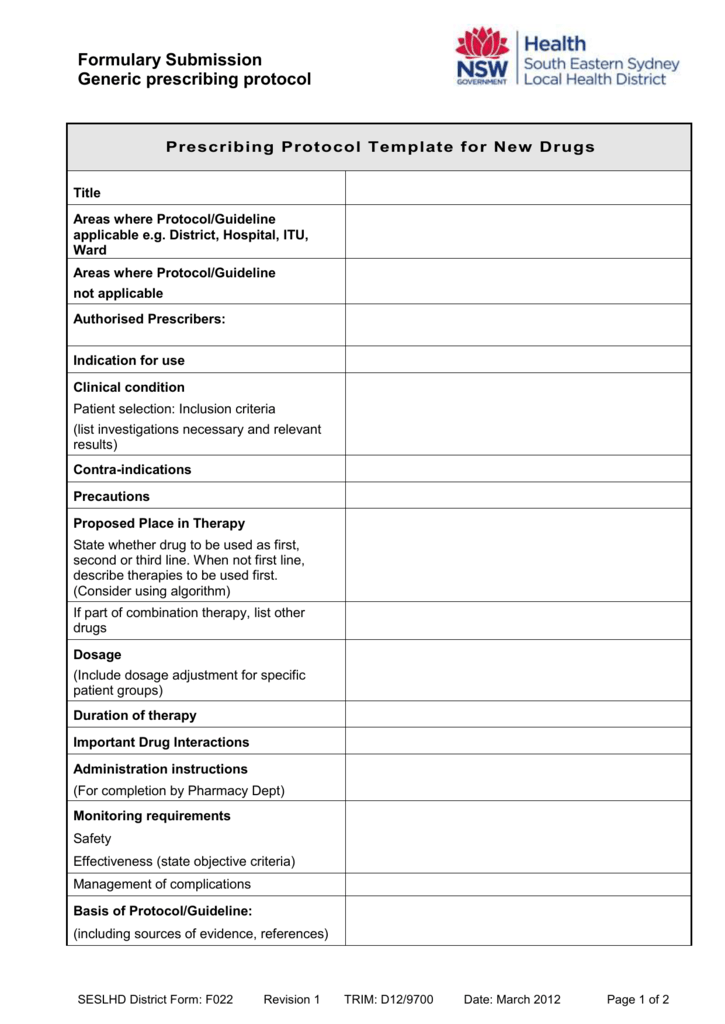
Prescribing Protocol Template for New Drugs
Protocol Template 05Feb2016 508 Clinical Trial Food And Drug
WA Health Research Protocol Template for Clinical Trials
Study Protocol Template Gambaran
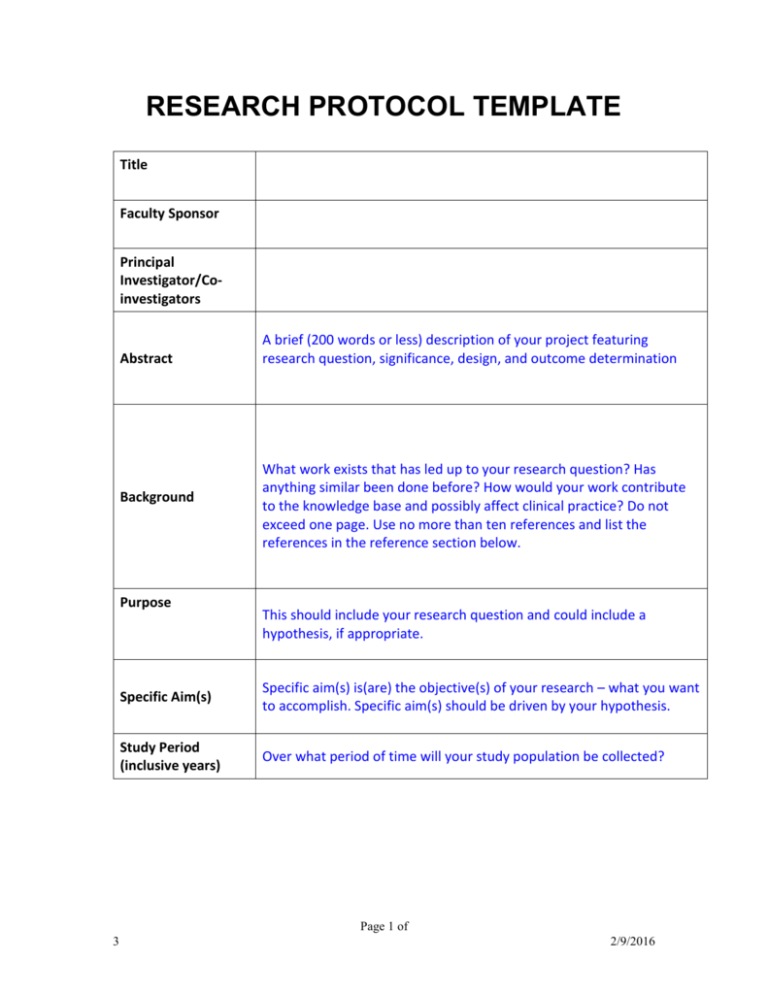
research protocol template
Clinical Trial Protocol Template Ema
Related Post: