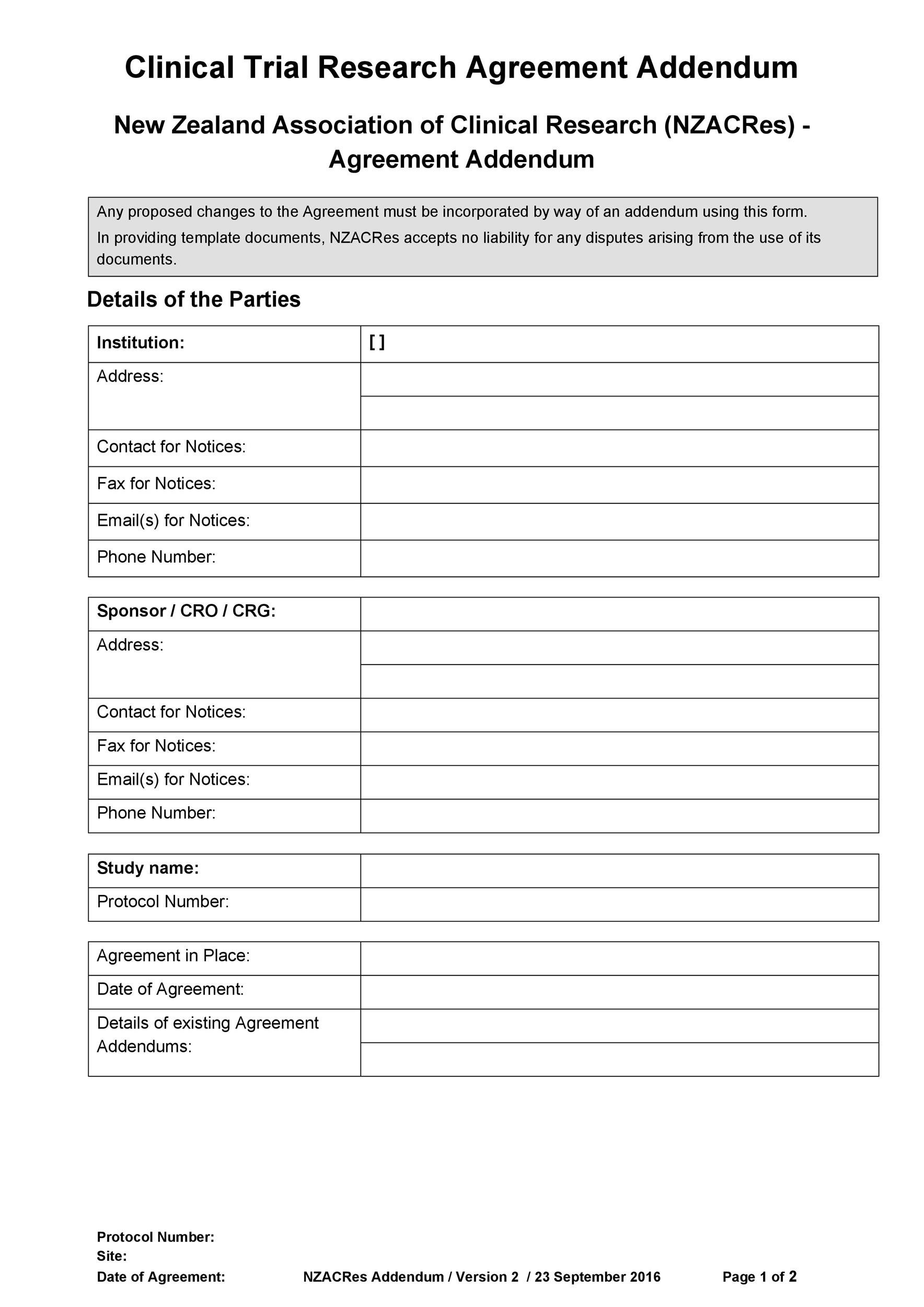
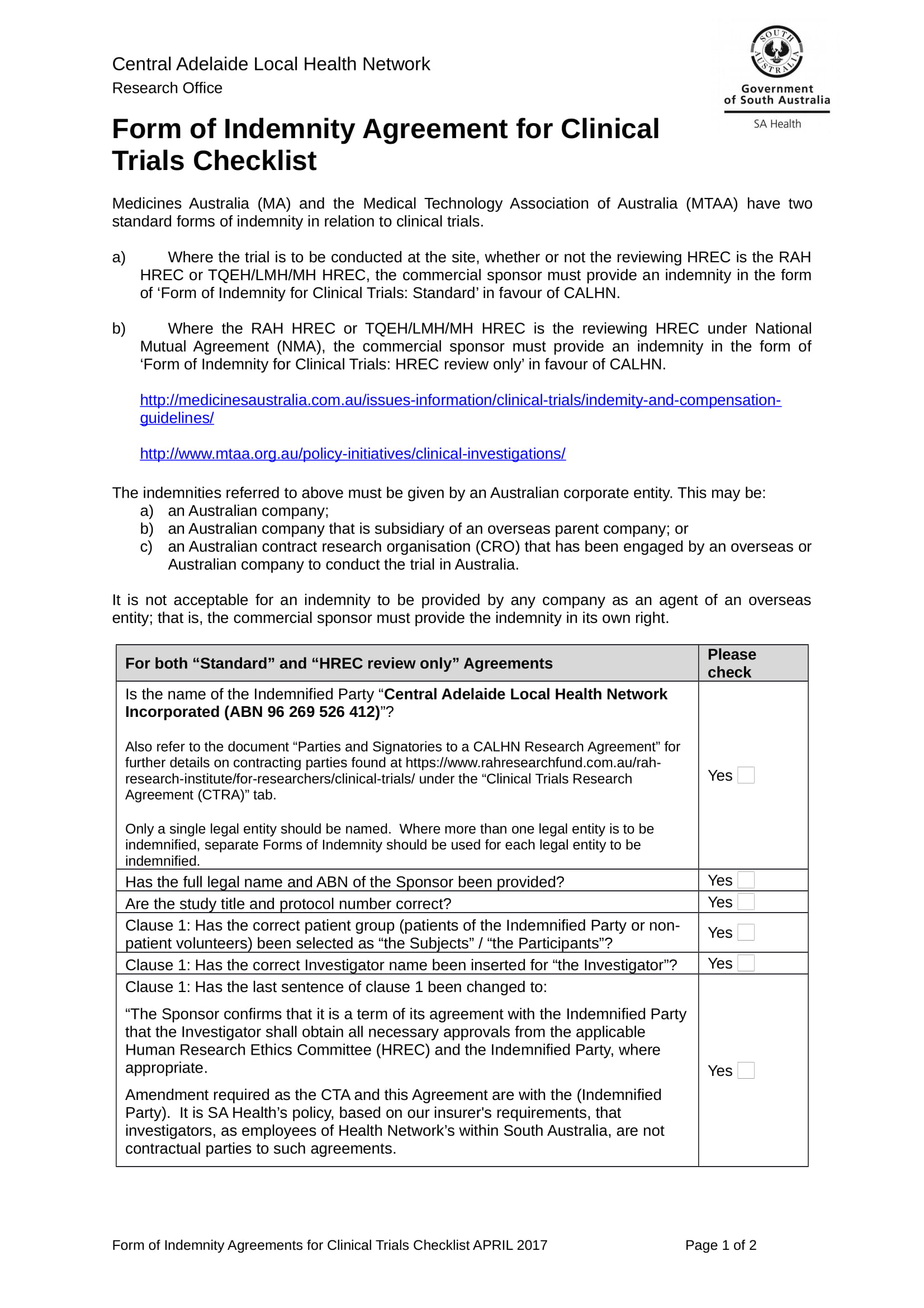
Clinical Trial Agreement Template
Clinical Trial Agreement Template - Agreement intake once a pi and sponsor have decided to pursue work on a clinical research project at the university of utah, the pi should notify the office of. Approved for use by the ctsa stakeholders, the nih, and the fdp. Final protocol date or version or investigational product number: Web the cta submission should include any documents provided by the funding partner for review or signature, but if a template agreement has not been provided,. Web use this free clinical trial agreement template to agree terms between sponsors and institutions in 2023. Web a clinical trial agreement (cta) is a legally binding agreement that manages the relationship between the sponsor that may be providing the study drug or device, the. Used for any study involving drugs/dietary supplements. Click on the image above to find out how you can try the full clinical. Web clinical trial agreements are vital for any clinical trial, establishing a legal contract between all parties involved and guaranteeing that regulatory requirements are met. Ad put the experience of our mobile research nurses to work for your trial. This template aims to facilitate the development of phase 2 and 3 clinical trial protocols that require a food. Used for any study involving drugs/dietary supplements. Used for any study involving medical devices (as defined by the fda ). Web the new zealand association of clinical research (nzacres) has developed a standardised clinical trial research agreement (sctra) based on the. Web notwithstanding anything contained herein to the contrary, if a subject fails to complete all study procedures, university shall be entitled to receive the larger of: Web clinical trial agreement us template version 11july2013 based on global template version 15april2013 page 2 of 28 site specific version november 2019 iqvia the. Web clinical trial agreements are vital for any clinical. Click on the image above to find out how you can try the full clinical. Ad put the experience of our mobile research nurses to work for your trial. Approved for use by the ctsa stakeholders, the nih, and the fdp. Web a clinical trial agreement (cta) is a legally binding agreement that manages the relationship between the sponsor that. Web clinical trial agreement us template version 11july2013 based on global template version 15april2013 page 2 of 28 site specific version november 2019 iqvia the. Web clinical trial research agreements. Number of clinical trial participants to be recruited for the clinical. Agreement intake once a pi and sponsor have decided to pursue work on a clinical research project at the. The pennsylvania state university and. Web the new zealand association of clinical research (nzacres) has developed a standardised clinical trial research agreement (sctra) based on the medicines. Used for any study involving drugs/dietary supplements. Web use this free clinical trial agreement template to agree terms between sponsors and institutions in 2023. Promptly following the date hereof, the parties shall negotiate. Web the new zealand association of clinical research (nzacres) has developed a standardised clinical trial research agreement (sctra) based on the medicines. Learn why we're a preferred mobile research provider for decentralized clinical trials. And _____ this clinical trial. Used for any study involving drugs/dietary supplements. Or disclose any individually identifiable health information attached to or contained within the biological. Used for any study involving medical devices (as defined by the fda ). The southern and eastern border states (forming the sebs panel) (comprising the nsw, qld, vic, sa and tas jurisdictions with act and. The pennsylvania state university and. Or disclose any individually identifiable health information attached to or contained within the biological. Web clinical trial agreements are vital. Agreement intake once a pi and sponsor have decided to pursue work on a clinical research project at the university of utah, the pi should notify the office of. Web agreements, approvals and contracts: This template aims to facilitate the development of phase 2 and 3 clinical trial protocols that require a food. Web clinical trial agreement us template version. The pennsylvania state university and. The southern and eastern border states (forming the sebs panel) (comprising the nsw, qld, vic, sa and tas jurisdictions with act and. Web the new zealand association of clinical research (nzacres) has developed a standardised clinical trial research agreement (sctra) based on the medicines. Approved for use by the ctsa stakeholders, the nih, and the. Web clinical trial agreements are vital for any clinical trial, establishing a legal contract between all parties involved and guaranteeing that regulatory requirements are met. And _____ this clinical trial. This template aims to facilitate the development of phase 2 and 3 clinical trial protocols that require a food. Web a clinical trial agreement (cta) is a legally binding agreement. Used for any study involving drugs/dietary supplements. Approved for use by the ctsa stakeholders, the nih, and the fdp. Final protocol date or version or investigational product number: Web clinical trial research agreements. Web agreements, approvals and contracts: Agreement intake once a pi and sponsor have decided to pursue work on a clinical research project at the university of utah, the pi should notify the office of. Web the cta submission should include any documents provided by the funding partner for review or signature, but if a template agreement has not been provided,. Number of clinical trial participants to be recruited for the clinical. Web clinical trial agreement us template version 11july2013 based on global template version 15april2013 page 2 of 28 site specific version november 2019 iqvia the. Clinical trial agreement (cta) with sponsors or contract research organisations (cros') sop. Learn why we're a preferred mobile research provider for decentralized clinical trials. Used for any study involving medical devices (as defined by the fda ). Promptly following the date hereof, the parties shall negotiate in good faith a clinical trial agreement on customary terms under which seller and its. The southern and eastern border states (forming the sebs panel) (comprising the nsw, qld, vic, sa and tas jurisdictions with act and. And _____ this clinical trial. Web notwithstanding anything contained herein to the contrary, if a subject fails to complete all study procedures, university shall be entitled to receive the larger of: Web use this free clinical trial agreement template to agree terms between sponsors and institutions in 2023. Web clinical trial agreements are vital for any clinical trial, establishing a legal contract between all parties involved and guaranteeing that regulatory requirements are met. Web a clinical trial agreement (cta) is a legally binding agreement that manages the relationship between the sponsor that may be providing the study drug or device, the. Or disclose any individually identifiable health information attached to or contained within the biological.TEMPLATE CLINICAL TRIAL AGREEMENT
Clinical Trial Agreement, Sample Clinical Trial Agreement Template
Free Investigator Initiated Clinical Trial Agreement Template Google
PPT The IOM Template Project Material Transfer Agreements and
NeoPharm, Inc. Master Clinical Trial Agreement for SponsorInitiated
Clinical study agreement template in Word and Pdf formats page 2 of 7
Clinical Trial Protocol Template Eu Templates NjQyMjk Resume Examples
CLINICAL TRIAL AGREEMENT FOR PHARMACEUTICAL
44 Professional Contract Amendment Templates & Samples ᐅ TemplateLab
FREE 5+ Indemnity Agreement Contract Forms in PDF MS Word
Related Post: