Clinical Monitoring Plan Template
Clinical Monitoring Plan Template - This template provides a recommended structure for developing consistent study procedure implementation and data collection instructions across participant and clinical. Web clinical site monitoring quality management overview if you are an nidcr grant applicant or awardee planning to conduct clinical research, you may need the following. Web clinical monitoring plan template describes how you will go about monitoring the conduct of your trial and justifies the approach taken. Web clinical site monitoring safety and problem event reporting (aes, deviations, unanticipated problems, & pregnancies) quality management screening & enrollment. Web this is an ms word template to use as a starting point for preparing a medical monitoring plan for clinical trials or research. Web the complete data and safety monitoring report template should be included as an appendix. Ad explore our interactive demo and experience the power of clinical trial analytics. Web clinical monitoring and data management plan (cmp/dmp) checklist this checklist is a tool to assist applicants and investigators in the development and writing of a cmp/dmp. Throughout the template there are. Web clinical monitoring plan for protocol: Web the national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to. Innovation for tomorrow begins with a partner you can trust today. Web clinical monitoring plan template describes how you will go about monitoring the conduct of your trial and justifies the approach taken. Web examples of. Web clinical trial templates to start your clinical research. Ad explore our interactive demo and experience the power of clinical trial analytics. Web clinical monitoring plan template describes how you will go about monitoring the conduct of your trial and justifies the approach taken. Also, we have included a proposed structure for a. This template provides a recommended structure for. It also outlines the responsibilities. Web this clinical monitoring plan (cmp) establishes the guidelines for conducting monitoring visits and related tasks for monitoring murdoch children’s research institute (mcri). Also, we have included a proposed structure for a. Web clinical monitoring plan template. Web ðï ࡱ á> þÿ ¯ þÿÿÿ«¬. Also, we have included a proposed structure for a. Web the national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to. Web clinical monitoring plan template. Web clinical monitoring plan for protocol: Web clinical study report template. Web clinical site monitoring quality management overview if you are an nidcr grant applicant or awardee planning to conduct clinical research, you may need the following. Ad powering research & development at the speed of science check out lifesphere by arisglobal. Web ðï ࡱ á> þÿ ¯ þÿÿÿ«¬. Web clinical trial templates to start your clinical research. Refer to. Web this is an ms word template to use as a starting point for preparing a medical monitoring plan for clinical trials or research. Revvity clinical solutions provides a holistic view across all your clinical data. Web ðï ࡱ á> þÿ ¯ þÿÿÿ«¬. Web clinical monitoring plan template. Web this template includes a proposed structure for a clinical monitoring. Refer to the niams data and safety monitoring board report templates (. Web clinical monitoring plan template. Ad explore our interactive demo and experience the power of clinical trial analytics. Web monitoring plan template instructions this template is a suggested format for a monitoring plan developed by tb survey teams. Ad powering research & development at the speed of science. Web details this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Web clinical monitoring plan template. Best practice recommendations review this draft. Budget monitoring tool with example data. Web nidcr clinical monitoring guidelines: Ad explore our interactive demo and experience the power of clinical trial analytics. Refer to the niams data and safety monitoring board report templates (. Best practice recommendations review this draft. Web clinical monitoring and data management plan (cmp/dmp) checklist this checklist is a tool to assist applicants and investigators in the development and writing of a cmp/dmp. Web the. Web clinical site monitoring quality management overview if you are an nidcr grant applicant or awardee planning to conduct clinical research, you may need the following. Web clinical monitoring plan template describes how you will go about monitoring the conduct of your trial and justifies the approach taken. Budget monitoring tool with example data. Web examples of studies requiring medium. Web this is an ms word template to use as a starting point for preparing a medical monitoring plan for clinical trials or research. Ad explore our interactive demo and experience the power of clinical trial analytics. Web this template includes a proposed structure for a clinical monitoring plan as well as draft. Web this clinical monitoring plan (cmp) establishes the guidelines for conducting monitoring visits and related tasks for monitoring murdoch children’s research institute (mcri). Ad put the experience of our mobile research nurses to work for your trial. Innovation for tomorrow begins with a partner you can trust today. Web clinical monitoring plan template describes how you will go about monitoring the conduct of your trial and justifies the approach taken. Best practice recommendations review this draft. Web ðï ࡱ á> þÿ ¯ þÿÿÿ«¬. Learn why we're a preferred mobile research provider for decentralized clinical trials. Web monitoring plan template instructions this template is a suggested format for a monitoring plan developed by tb survey teams. Web clinical study report template. Web clinical monitoring plan for protocol: Web clinical site monitoring safety and problem event reporting (aes, deviations, unanticipated problems, & pregnancies) quality management screening & enrollment. Web clinical site monitoring quality management overview if you are an nidcr grant applicant or awardee planning to conduct clinical research, you may need the following. Web nidcr clinical monitoring guidelines: This template provides a recommended structure for developing consistent study procedure implementation and data collection instructions across participant and clinical. Guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Web clinical trial templates to start your clinical research. Web the national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to.Individual Monitoring Plan Template
Free Clinical Trial Templates Smartsheet
Monitoring Visit Report Template Tools & Resources
Monitoring Report Template Clinical Trials ] Saving Lives Pertaining
Monitoring Plan Template Tools & Resources
Monitoring Plan Template
Monitoring Plan PDF Clinical Trial Pharmacy
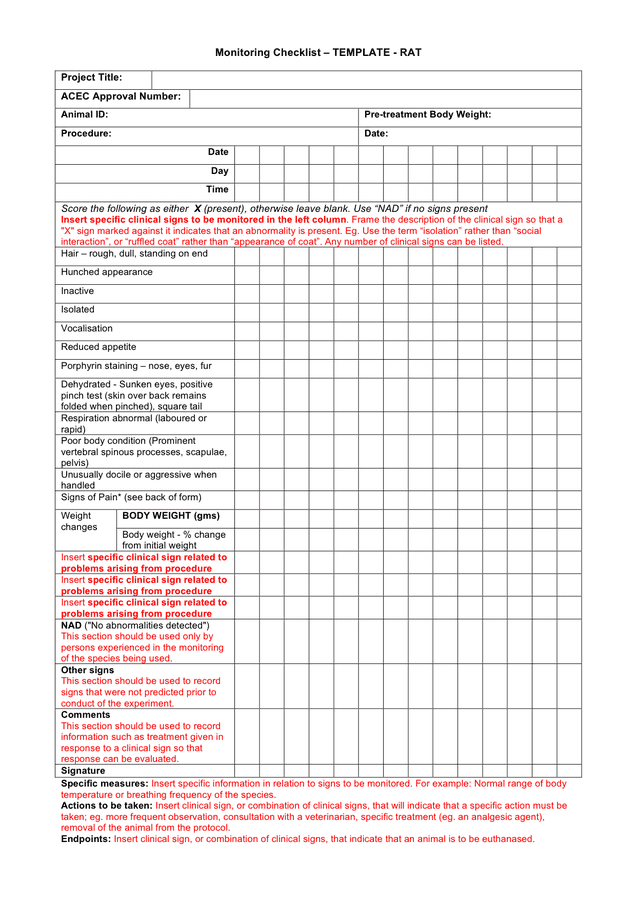
Monitoring checklist template in Word and Pdf formats
Monitoring Report Template Clinical Trials (5) TEMPLATES EXAMPLE
Patient Monitoring Sheet PDF Diseases And Disorders Health Care
Related Post:



![Monitoring Report Template Clinical Trials ] Saving Lives Pertaining](https://pray.gelorailmu.com/wp-content/uploads/2020/01/monitoring-report-template-clinical-trials-saving-lives-pertaining-to-monitoring-report-template-clinical-trials-1603x2048.png)