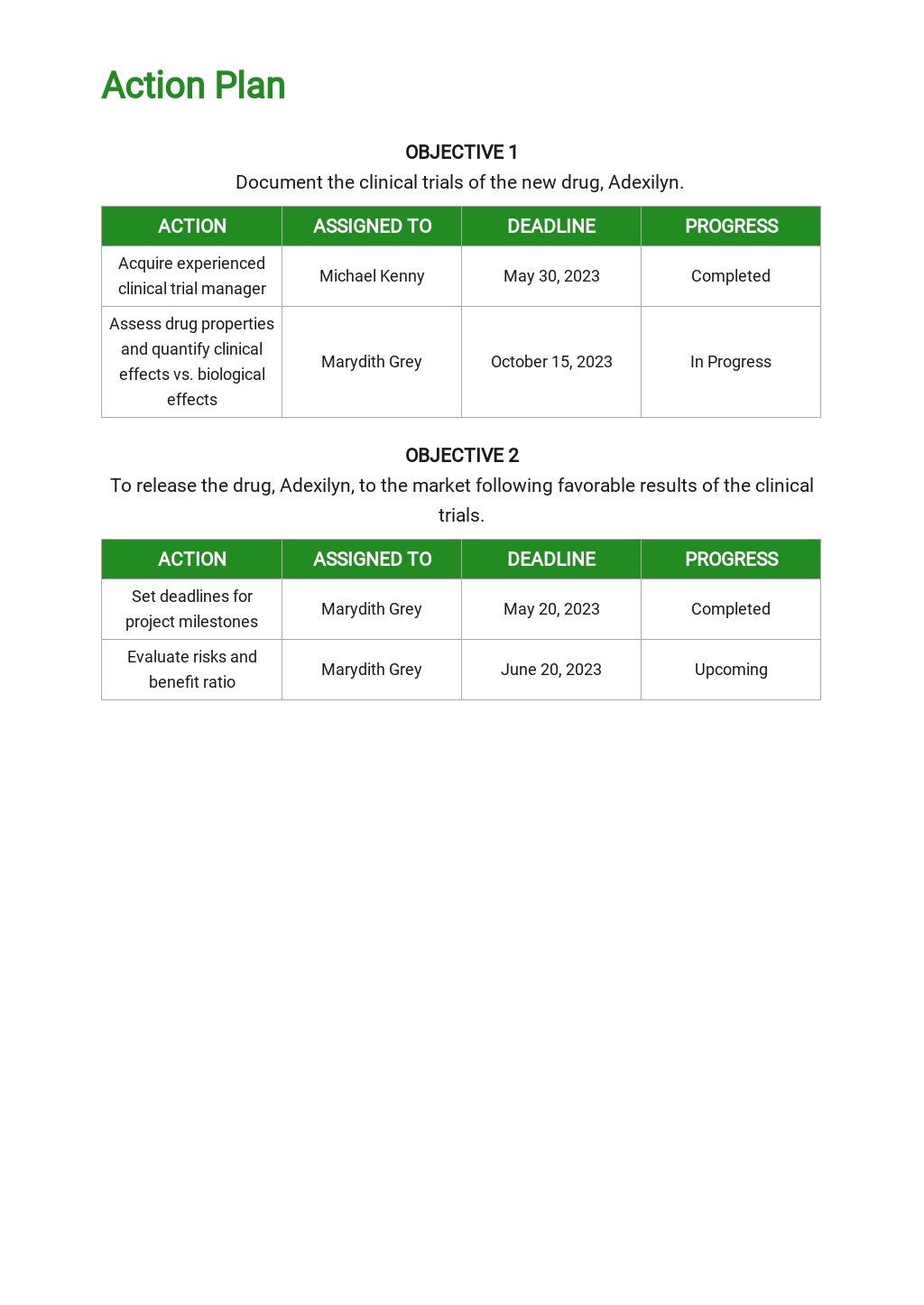
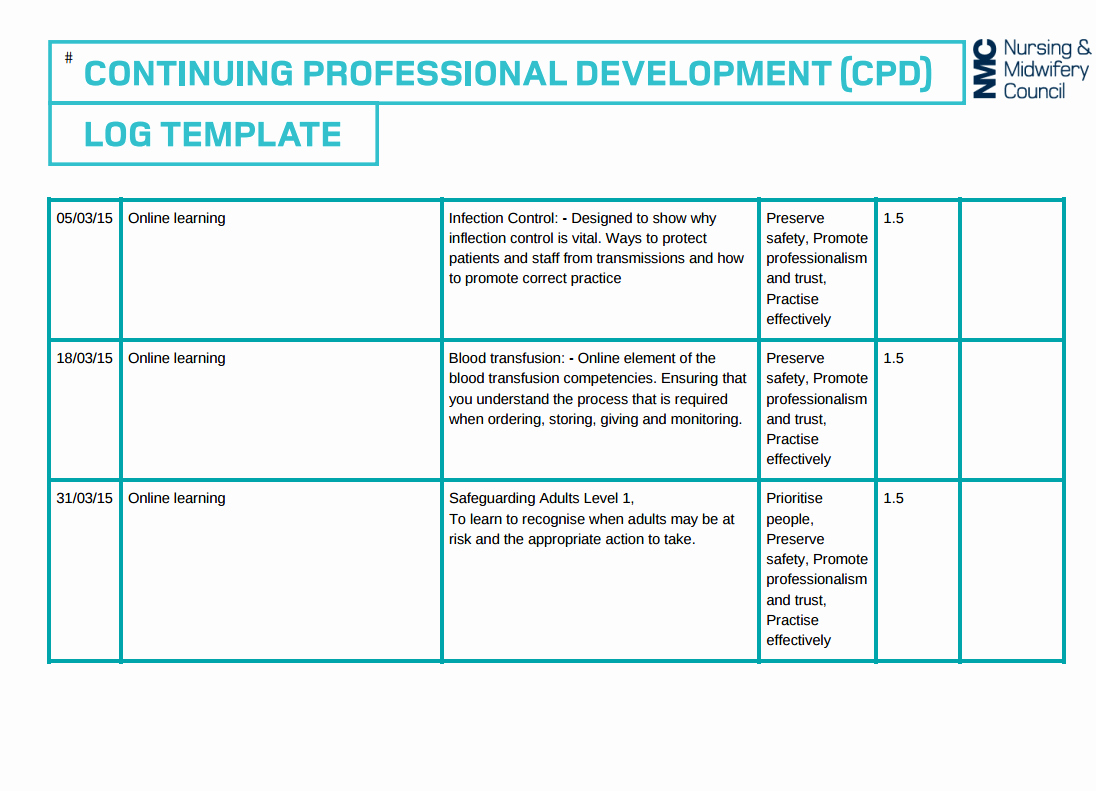
Clinical Development Plan Template
Clinical Development Plan Template - Simplify treatment planning with a fully integrated and customizable template library. Web the clinical development plan is the blueprint of the entire clinical research strategy of a drug which defines the critical path for the clinical program including development assessment and decision points and the project resource (personnel and. What is the role of a. Departments should share content for their department. Ad 3m cdi solutions use shared workflows to improve documentation and quality scores. Cdi software from 3m uses ai and integrated workflows to achieve accurate documentation. Some manufacturers may need more clarity, though, in order to meet cdp requirements as. Annex xiv of the medical devices regulation (eu) 2017/745 (mdr), section 1 (a), 8 th indent, requires a clinical development plan (cdp). Web the following table includes explanations of various components of an ind application and links to additional information related to application submission. Regional meeting budget template with example data. The clinical development plan is the blueprint of the entire clinical research strategy of a drug, which defines the critical path for the clinical program, including development, assessment, and decision points, and the. (*clinical development plan is initiated prior to the fih gate review and is updated & reviewed during development through to the dtf gate review. Web develop your. It outlines the clinical program design, including development, assessment, decision points, personnel, and budgetary estimates. The cdp is the blueprint of a drug’s entire clinical research strategy. Web develop your own communication plan using this free clinical trial communication plan template. This template also includes a section for situation analysis and risk analysis that asks for inputs on strengths, weaknesses,. Simplify treatment planning with a fully integrated and customizable template library. This template also includes a section for situation analysis and risk analysis that asks for inputs on strengths, weaknesses, opportunities, and. Cdi software from 3m uses ai and integrated workflows to achieve accurate documentation. Web the following table includes explanations of various components of an ind application and links. Annex xiv of the medical devices regulation (eu) 2017/745 (mdr), section 1 (a), 8 th indent, requires a clinical development plan (cdp). The cdp is the blueprint of a drug’s entire clinical research strategy. Ad 3m cdi solutions use shared workflows to improve documentation and quality scores. Web the following table includes explanations of various components of an ind application. Web * faculty below are listed as examples. Ad put the experience of our mobile research nurses to work for your trial. Our templates have been completely updated to reflect the many recent requirements. Regional meeting budget template with example data. Simplify treatment planning with a fully integrated and customizable template library. Web the clinical development plan is the blueprint of the entire clinical research strategy of a drug which defines the critical path for the clinical program including development assessment and decision points and the project resource (personnel and. Web clinical development plan (cdp). Our templates have been completely updated to reflect the many recent requirements. Regional meeting budget template with. What is the role of a. Web clinical development plan (cdp) design. Web develop your own communication plan using this free clinical trial communication plan template. The vision is transformed into distinct implementation phases and discrete steps, called clinical studies, each with well defined. Web clinical development plan. This template also includes a section for situation analysis and risk analysis that asks for inputs on strengths, weaknesses, opportunities, and. Web develop your own communication plan using this free clinical trial communication plan template. Annex xiv of the medical devices regulation (eu) 2017/745 (mdr), section 1 (a), 8 th indent, requires a clinical development plan (cdp). Our templates have. Web the following table includes explanations of various components of an ind application and links to additional information related to application submission. Web this white paper provides an overview of creating an integrated drug development plan, an important tool for identifying development challenges and devising strategies that increase the likelihood of delivering a new, approved medicine to patients. Web clinical. Web the medical device regulation (mdr) mentions the term “clinical development plan” (cdp) only twice, both of which are in than the mdr into what the cdp entails and to propose the best strategies for a manufacturer to plan their medical device’s clinical. Web this white paper provides an overview of creating an integrated drug development plan, an important tool. Web the following table includes explanations of various components of an ind application and links to additional information related to application submission. Cdi software from 3m uses ai and integrated workflows to achieve accurate documentation. Web the medical device regulation (mdr) mentions the term “clinical development plan” (cdp) only twice, both of which are in than the mdr into what the cdp entails and to propose the best strategies for a manufacturer to plan their medical device’s clinical. Our network of specialized laboratories, each bringing distinct expertise and perspectives, collaborates in a unified effort to discover and develop effective treatments. Clinical development project management plan. Web clinical development plan. Web clinical safety data and rationale for phase 2 dose selection. (*clinical development plan is initiated prior to the fih gate review and is updated & reviewed during development through to the dtf gate review. Identifying critical suppliers sop : It outlines the clinical program design, including development, assessment, decision points, personnel, and budgetary estimates. Web a clinical development plan — a comprehensive strategy for developing an investigational product through regulatory submission — is a critical component of drug development and helps ensure that new therapies are safe, effective, and of high. Some manufacturers may need more clarity, though, in order to meet cdp requirements as. Ad 3m cdi solutions use shared workflows to improve documentation and quality scores. Web this white paper provides an overview of creating an integrated drug development plan, an important tool for identifying development challenges and devising strategies that increase the likelihood of delivering a new, approved medicine to patients. Web clinical development plan (cdp) design. Based on meddev 2.7/1 rev. Our templates have been completely updated to reflect the many recent requirements. What is the role of a. Web clinical development plan (cdp). Departments should share content for their department.Clinical Development Plan Template Luxury Patient Engagement Strategies
Clinical Development Plan Template
Clinical Development Plan Template in Google Docs, Word, Apple Pages
Clinical Development Plan Template in Google Docs, Word, Apple Pages
Clinical Development Plan Template Venngage
Clinical Development Plan Template Google Docs, Word, Apple Pages
Clinical Development Plan Template
Clinical Trials Strategy The Clinical Development Plan
Clinical Development Plan Template
Clinical Development Plan Template
Related Post: