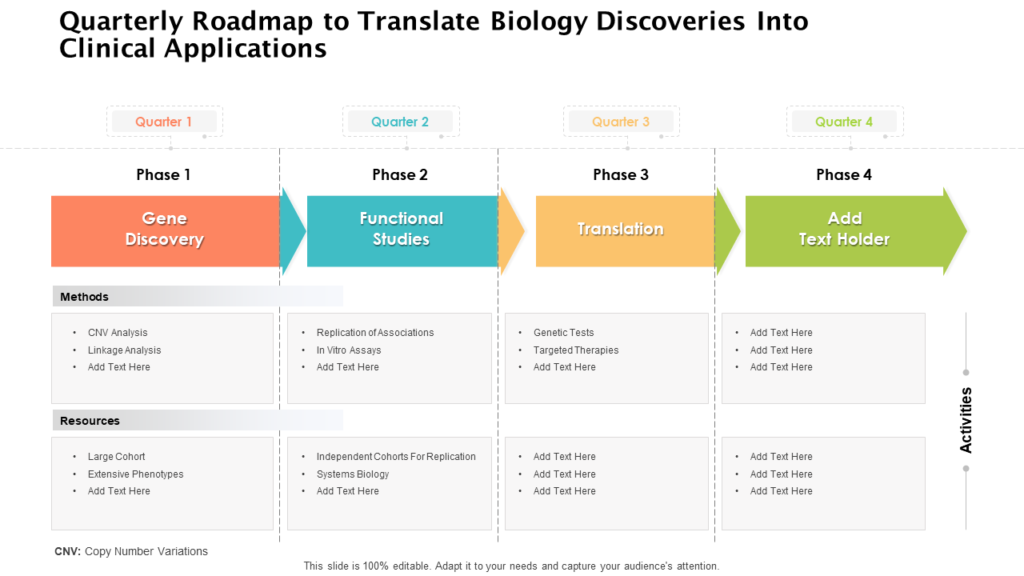
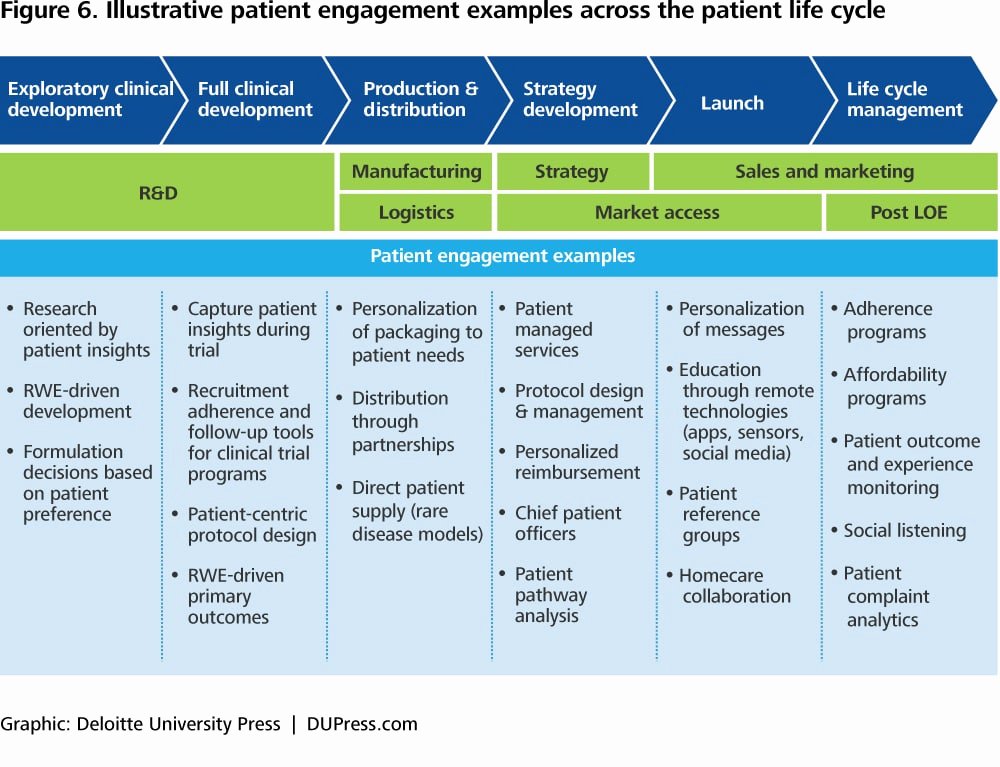
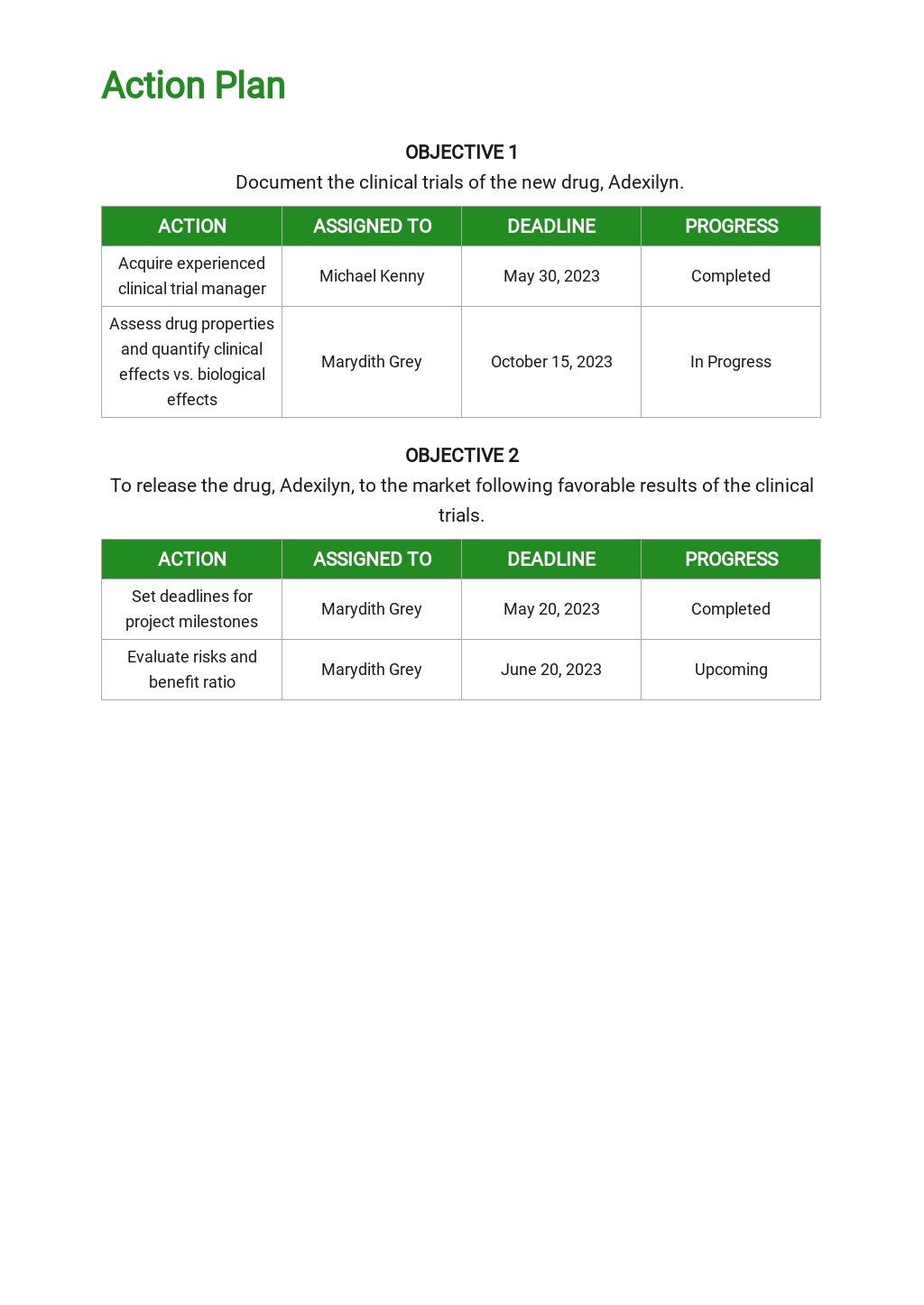
Clinical Development Plan Template Fda
Clinical Development Plan Template Fda - Web regulatory bodies such as federal drug association (fda), european medicines agency (ema) and the uk’s medicines and healthcare products regulatory agency have. Web clinical development plan (cdp) design. Web this section is intended to place the clinical development plan for the investigational new drug into perspective and to help fda anticipate the needs of the. Web this protocol template aims to facilitate the development of two types of clinical trials involving human participants. The first type of trials are phase 2 and 3. Web a target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidelines. The cber office of cellular, tissue and gene therapies. Interactive visualizations & predictive analytics allows you to explore clinical data. Project management, site management, data management, regulatory insight services & more! Web download now clinical trails comprehensive development plan download now data standardization planning for clinical development programs download now clinical. Web welcome to the october issue of going on trial, a monthly newsletter covering clinical trials and drug development for autism and related conditions. A document describing the clinical studies that must be performed during the development of a particular active substance, device, procedure. Web clinical development plan • generate cdp scenarios • timelines and key milestones • endpoint strategy. Web download now clinical trails comprehensive development plan download now data standardization planning for clinical development programs download now clinical. Web clinical development plan • generate cdp scenarios • timelines and key milestones • endpoint strategy • regulatory strategy • statistical modeling • study design concept. Web clinical development plan extends beyond dtf to accommodate the time needed to report. Web clinical development plan (cdp) design. Web a target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidelines. Web a clinical development plan — a comprehensive strategy for developing an investigational product through regulatory submission — is a critical component of drug. Web 137 rows clinical trials guidance documents | fda clinical trials guidance. Web. Ad impactful performance reviews that drive success and growth. Help employees take their career development to the next level with our free template. Web 137 rows clinical trials guidance documents | fda clinical trials guidance. Web this protocol template aims to facilitate the development of two types of clinical trials involving human participants. 1) the explicit willingness to accept an. Help employees take their career development to the next level with our free template. Web the clinical development plan guide there are two essential documents that any company developing a new pharmaceutical or biotechnology derived product requires to. Web clinical development plan extends beyond dtf to accommodate the time needed to report phase 3 results and also cover additional plans. The first type of trials are phase 2 and 3. Interactive visualizations & predictive analytics allows you to explore clinical data. Web project catalyst’s oncology regulatory expertise and early guidance (oreeg) is an educational initiative of the oncology center of excellence (oce),. The clinical development plan is the blueprint of the entire clinical research strategy of a drug, which defines. Ad impactful performance reviews that drive success and growth. Web what is a clinical development strategic plan? Web this section is intended to place the clinical development plan for the investigational new drug into perspective and to help fda anticipate the needs of the. Interactive visualizations & predictive analytics allows you to explore clinical data. 1) the explicit willingness to. Web the clinical development plan is the blueprint of the entire clinical research strategy of a drug which defines the critical path for the clinical program including. Ad impactful performance reviews that drive success and growth. Ad impactful performance reviews that drive success and growth. Web the fda has qualified mddts for a wide range of device types such as. Project management, site management, data management, regulatory insight services & more! It outlines the clinical program design, including. Web this section is intended to place the clinical development plan for the investigational new drug into perspective and to help fda anticipate the needs of the. Interactive visualizations & predictive analytics allows you to explore clinical data. Ad revvity clinical solutions. The first type of trials are phase 2 and 3. Interactive visualizations & predictive analytics allows you to explore clinical data. Choose from thousands of templates like treatment plans, interventions, and more. Web download now clinical trails comprehensive development plan download now data standardization planning for clinical development programs download now clinical. Help employees take their career development to the. Ad impactful performance reviews that drive success and growth. Project management, site management, data management, regulatory insight services & more! Web this section is intended to place the clinical development plan for the investigational new drug into perspective and to help fda anticipate the needs of the. Web welcome to the october issue of going on trial, a monthly newsletter covering clinical trials and drug development for autism and related conditions. Web a clinical development plan — a comprehensive strategy for developing an investigational product through regulatory submission — is a critical component of drug. Web the fda has qualified mddts for a wide range of device types such as cardiovascular, neurology, ophthalmology, plastic surgery, automated insulin dosing, and. Web the most significant clarifications are: Web clinical development plan (cdp) design. Web this protocol template aims to facilitate the development of two types of clinical trials involving human participants. Web regulatory bodies such as federal drug association (fda), european medicines agency (ema) and the uk’s medicines and healthcare products regulatory agency have. A document describing the clinical studies that must be performed during the development of a particular active substance, device, procedure. Web download now clinical trails comprehensive development plan download now data standardization planning for clinical development programs download now clinical. Web 137 rows clinical trials guidance documents | fda clinical trials guidance. Ad revvity clinical solutions provides a holistic view across all your clinical data. The first type of trials are phase 2 and 3. Help employees take their career development to the next level with our free template. Help employees take their career development to the next level with our free template. Web the clinical development plan shall include (a) an outline of clinical trials to be conducted by zai in the territory, including the local studies and joint global studies;. Web the clinical development plan is a prerequisite for clinical evaluation, where manufacturers make a strategy for clinical evaluation based on medical device. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted.Top 20 PowerPoint Templates to Create a Clinical Development Plan
30 Clinical Development Plan Template Hamiltonplastering
Vanda Pharmaceuticals Inc. FORM 8K EX99.1 March 9, 2010
Understanding the 5 Phases of Medical Device Development
Clinical Development Plan Template Venngage
Clinical Development Plan Template Google Docs, Word, Apple Pages
Clinical Trials Strategy The Clinical Development Plan
Clinical Development Plan Template in Google Docs, Word, Apple Pages
Clinical Development Plan Template Fresh Session 3 Part 2 How to plan
Clinical Development Plan Template in Google Docs, Word, Apple Pages
Related Post: