Capa Template
Capa Template - A process to begin, investigate, and apply a corrective action plan. Web this template provides a detailed guide on how to implement and track capas according to iso 13485:2016. Web a capa plan is a corrective and preventive action plan, designed to identify and rectify issues and ensure they aren’t repeated. Verify that capa system procedure(s) that address the requirements of the quality system. Web how to fill up the capa format quickly? Web capa template the following is a template for the content and format of a capa to the study investigators. Web capa report form template [free download] digitize and automate capa reporting processes what is a corrective and preventive action (capa) report a. Web corrective and preventive action, sometimes referred to as capa, is a quality management strategy that is made up of processes that intend to correct and prevent. Web download corrective and preventative action plan form template_2019.11.13. [date that the capa is written] to:. Stay organized and get an overview of your projects & goals with the action plan template. How do i create a capa plan? Web how to fill up the capa format quickly? Web this article covers the important differences between corrective action and preventive action. Web corrective and preventive action, sometimes referred to as capa, is a quality management strategy. Web download corrective and preventative action plan form template_2019.11.13. Web this template provides a detailed guide on how to implement and track capas according to iso 13485:2016. Corrective action and preventive action (capa) plan template. Web actions (capa) plans guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying deviation patterns and areas of noncompliance. Web capa report form. Take immediate corrective actions if you become aware of a deviation or unexpected event that endangers the rights, welfare, or safety of participants and others,. Clear establishment of the issues that require this plan. Web you can download our free capa workflow template here, or continue reading to learn why a well formulated capa workflow is so important for closing. Web a capa plan is a corrective and preventive action plan, designed to identify and rectify issues and ensure they aren’t repeated. Web download corrective and preventative action plan form template_2019.11.13. Web this article covers the important differences between corrective action and preventive action. How do i create a capa plan? Web capa template the following is a template for. Web you can download our free capa workflow template here, or continue reading to learn why a well formulated capa workflow is so important for closing out a. Web capa to the irb protocol deviation report form (example) •this form is now a smart form submitted in eirb as part of the reportable event along with other smart form pages.. Web capa report form template [free download] digitize and automate capa reporting processes what is a corrective and preventive action (capa) report a. Web you can download our free capa workflow template here, or continue reading to learn why a well formulated capa workflow is so important for closing out a. Web download corrective and preventative action plan form template_2019.11.13.. Web this article covers the important differences between corrective action and preventive action. Web download corrective and preventative action plan form template_2019.11.13. Web corrective and preventive actions (capa) inspectional objectives. Web ultimately, an eqms could simplify internal and external (fda) audits of the capa system, and ensure no steps are missed along the way. Your corrective action plan template must. Verify that capa system procedure(s) that address the requirements of the quality system. Want to make capa and non. Corrective action and preventive action (capa) plan template. Web download corrective and preventative action plan form template_2019.11.13. Web this article covers the important differences between corrective action and preventive action. Clarification of contractor or team member responsibilities. Web ultimately, an eqms could simplify internal and external (fda) audits of the capa system, and ensure no steps are missed along the way. Take immediate corrective actions if you become aware of a deviation or unexpected event that endangers the rights, welfare, or safety of participants and others,. Web a capa plan. Corrective action and preventive action (capa) plan template. Web this article covers the important differences between corrective action and preventive action. Web corrective and preventive actions (capa) inspectional objectives. Your corrective action plan template must include: A standard way of dealing with deficiencies. It discusses capa within iso 9001 and within the regulation fda 21. Web this template provides a detailed guide on how to implement and track capas according to iso 13485:2016. It covers the input, output, process steps, participants, and verification. Clarification of contractor or team member responsibilities. [date that the capa is written] to:. Corrective action and preventive action (capa) plan template. Web actions (capa) plans guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying deviation patterns and areas of noncompliance. Web ultimately, an eqms could simplify internal and external (fda) audits of the capa system, and ensure no steps are missed along the way. Take immediate corrective actions if you become aware of a deviation or unexpected event that endangers the rights, welfare, or safety of participants and others,. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Web capa to the irb protocol deviation report form (example) •this form is now a smart form submitted in eirb as part of the reportable event along with other smart form pages. A standard way of dealing with deficiencies. Web corrective and preventive actions (capa) inspectional objectives. Web how to fill up the capa format quickly? Web you can download our free capa workflow template here, or continue reading to learn why a well formulated capa workflow is so important for closing out a. A process to begin, investigate, and apply a corrective action plan. Web this article covers the important differences between corrective action and preventive action. Want to make capa and non. Web download corrective and preventative action plan form template_2019.11.13. Web a capa plan is a corrective and preventive action plan, designed to identify and rectify issues and ensure they aren’t repeated.Sample Capa Form
CAPA form Corrective action and preventive action
Capa Form Template Free Printable Form, Templates and Letter
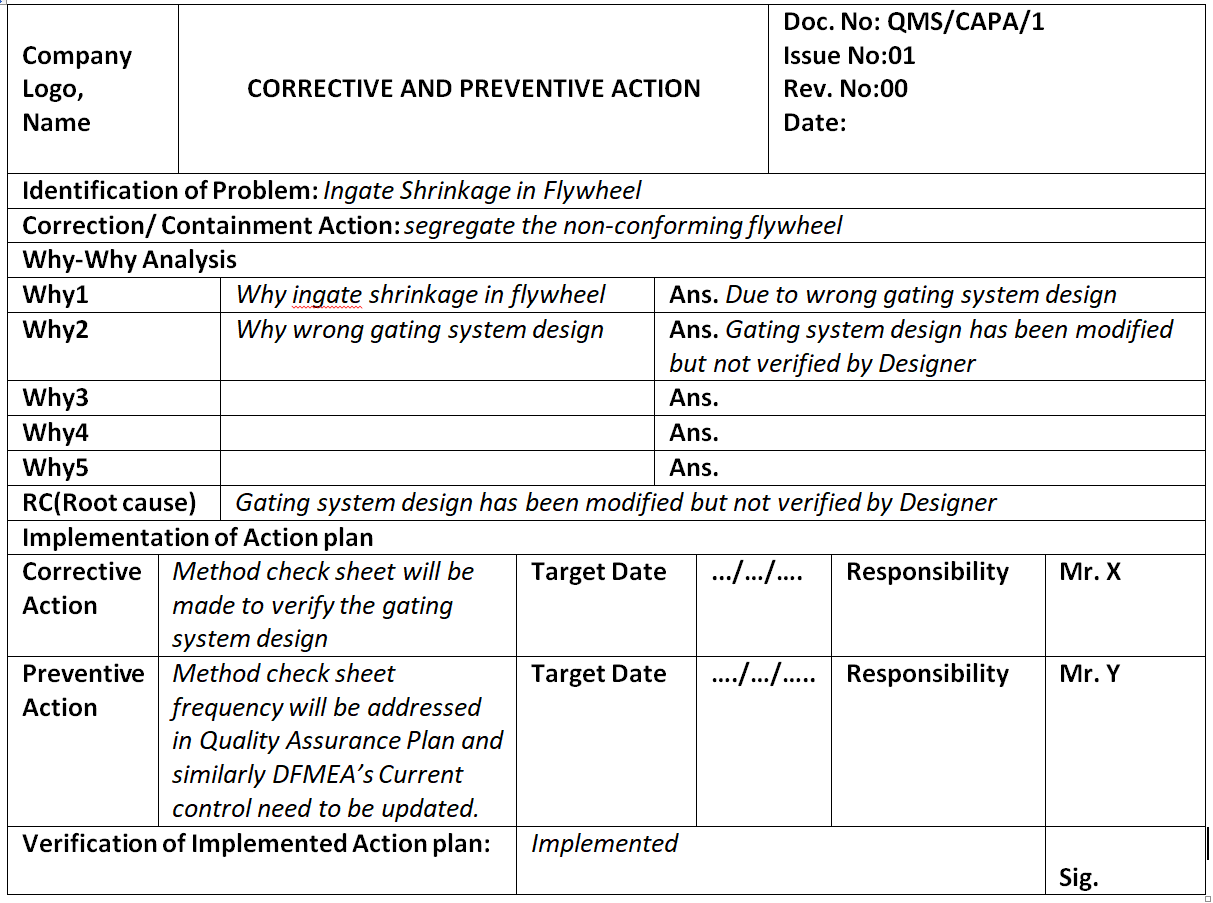
Corrective and Preventive Action Format CAPA with Example
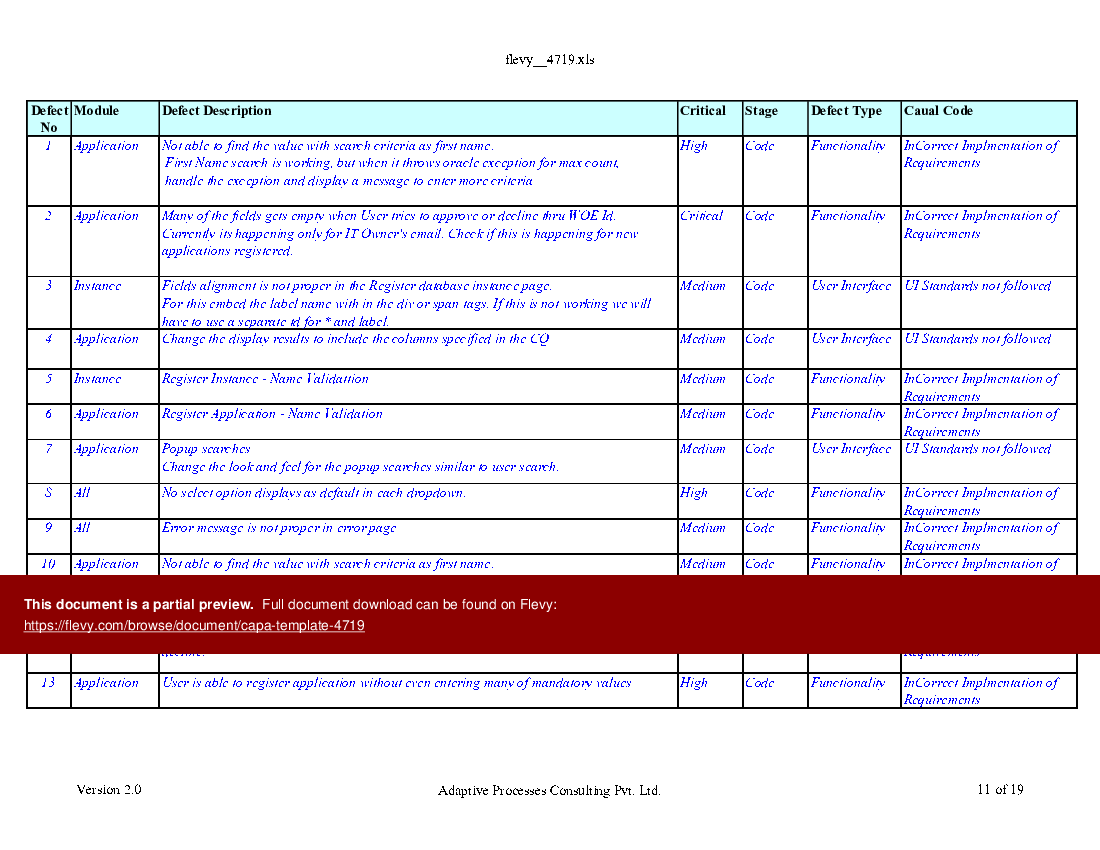
CAPA Template (Excel workbook (XLS)) Flevy
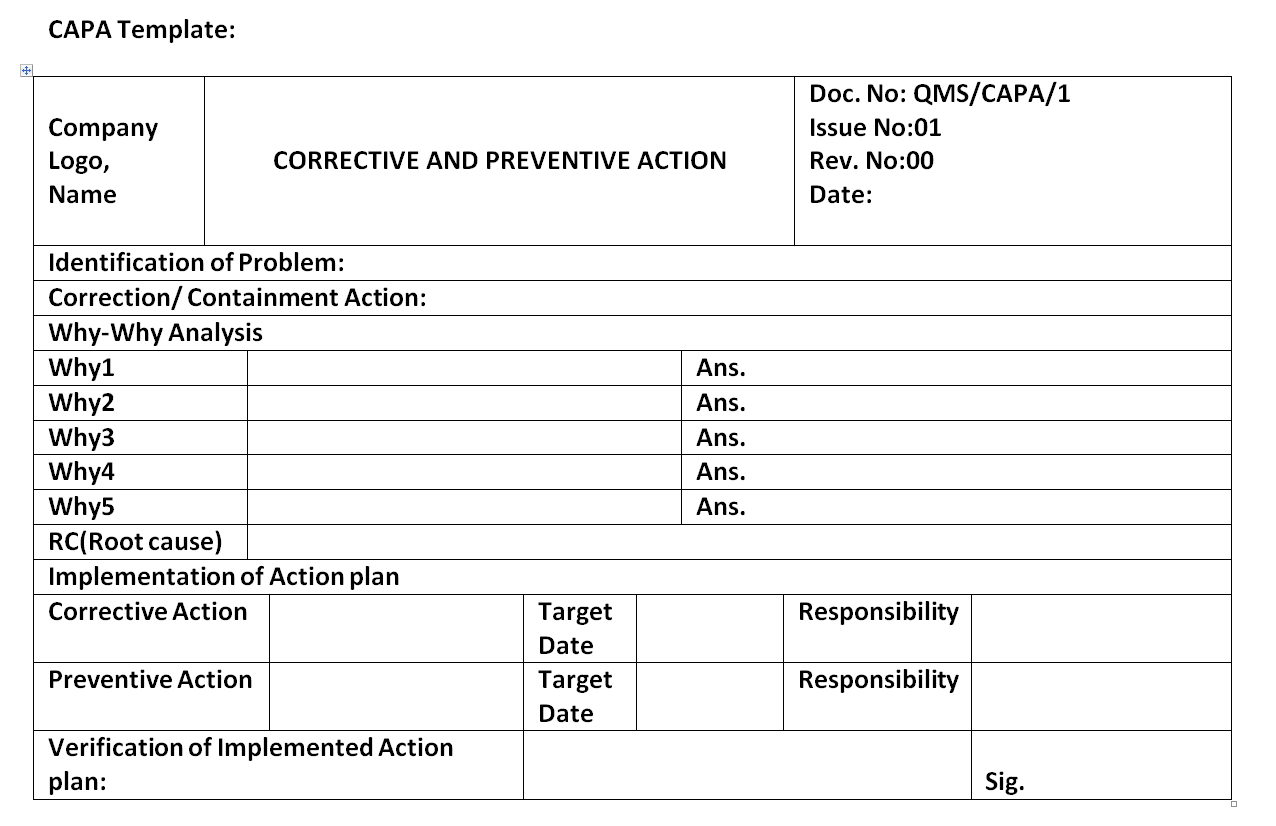
Corrective and Preventive Action Format CAPA with Example
Capa Form Template Free Printable Form, Templates and Letter
Sample Capa Form
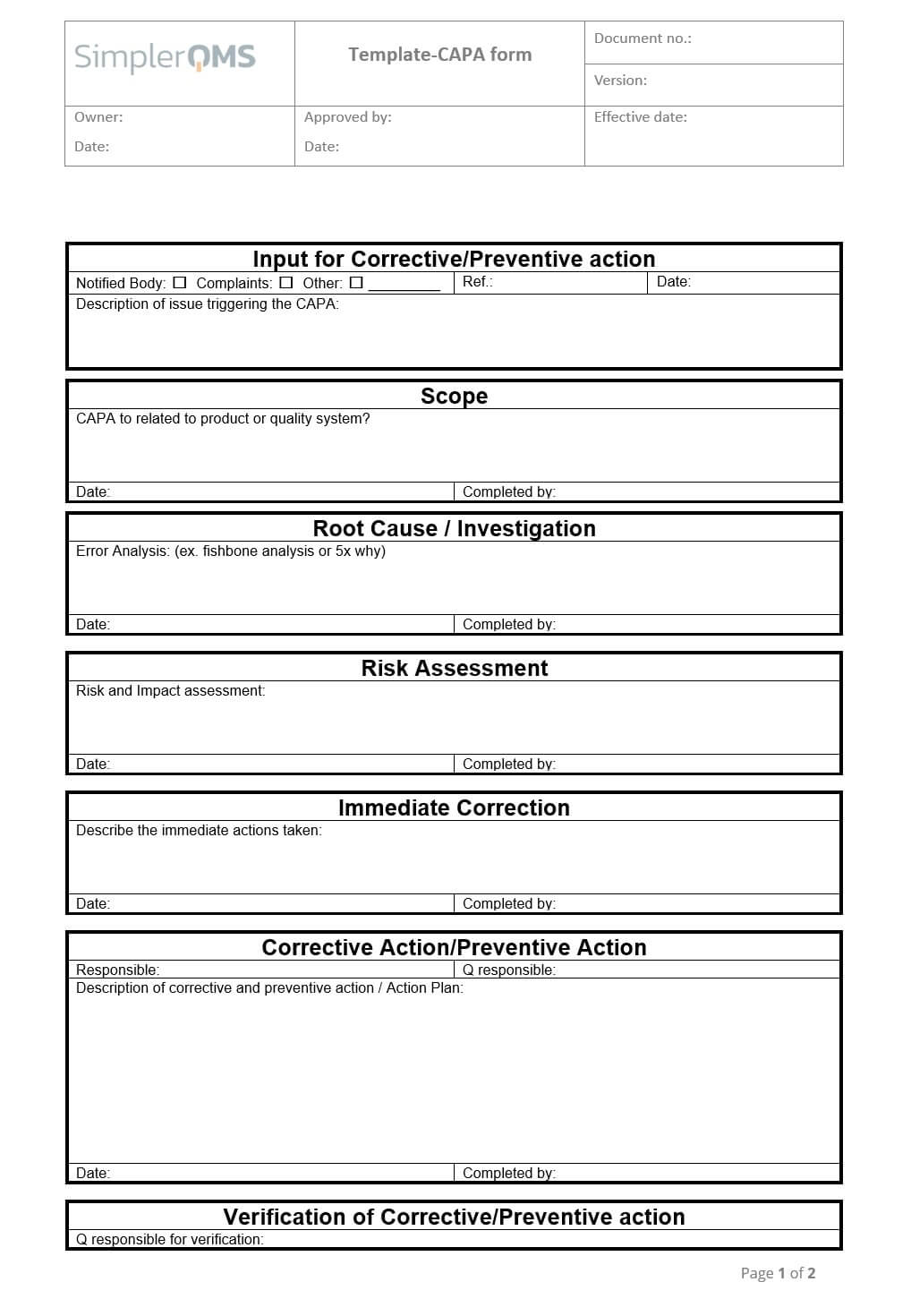
CAPA Form Example
CAPAform
Related Post: