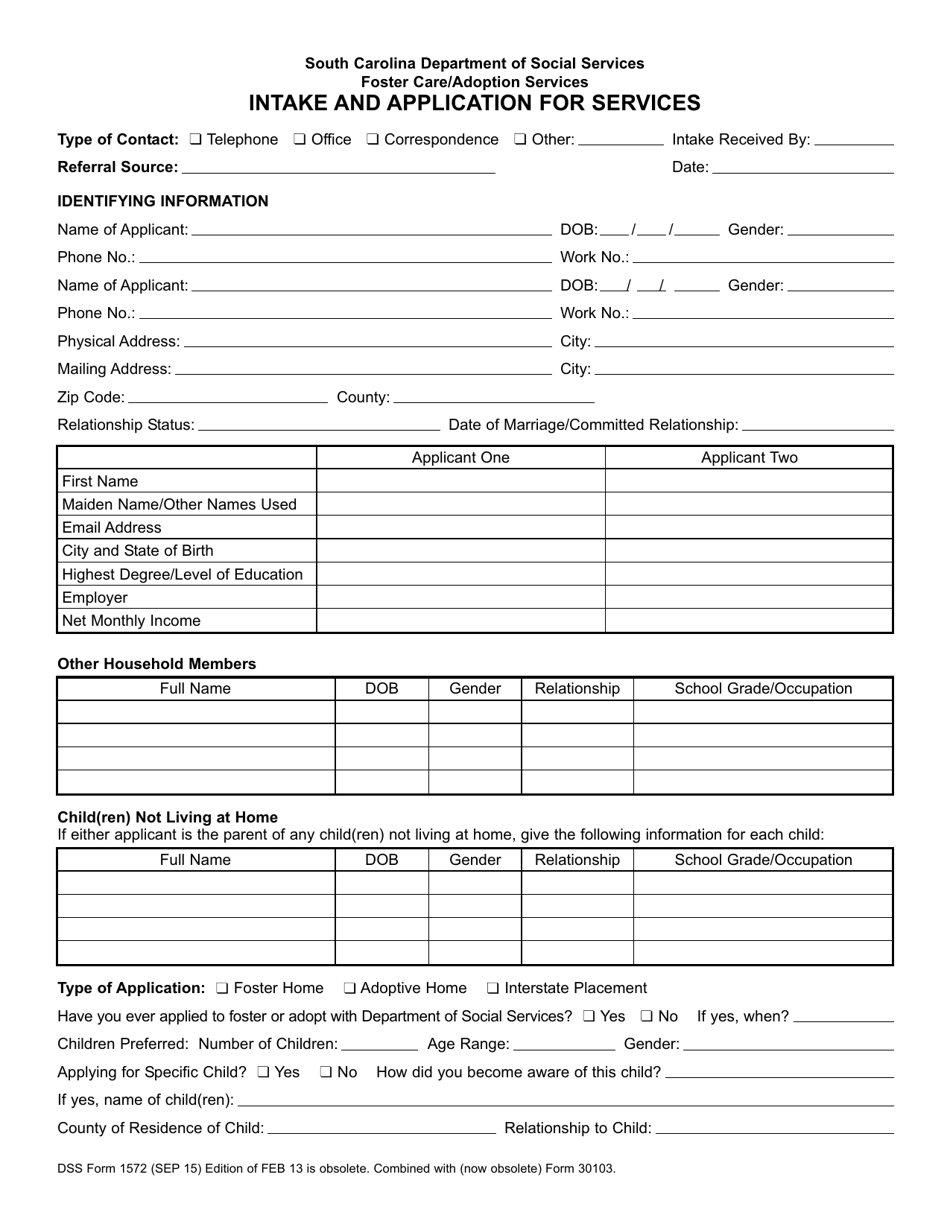
1572 Template
1572 Template - Web instructions for forms fda's receipt of the ind forms: 1) to provide the sponsor with information about the investigator’s qualifications and the clinical site that will enable the sponsor to establish Web the statement of investigator form fda 1572 is an agreement signed by the investigator to provide certain information to the sponsor and assure that he/she will comply with fda regulations related to the conduct of a clinical investigation of an investigational drug or biologic. Web the 1572 has two purposes: The commitments the investigator agrees to by signing form fda 1572. Web it describes how to complete the statement of investigator form (form fda 1572). Web fda releases draft guidance about form fda 1572. There are dozens of gift certificate templates over at canva. Web check out our 72 in stole template selection for the very best in unique or custom, handmade pieces from our paper shops. What the purpose of form fda 1572 is. Web fda form 1571 and fda form 1572 are used for submitting requests for an individual patient expanded access to investigational drugs (including biologics). Next, point and click through the steps of creating your custom. Web start by choosing your favorite template from our online library of 5 x 7 wedding invitation cards design templates. When form fda 1572 is. Web follow the simple instructions below: Ad signnow allows users to edit, sign, fill & share all type of documents online. On 20 may 2021, the fda released a draft information sheet guidance for sponsors, clinical investigators, and. Food and drug administration’s (fda’s) form fda 1572 is one of the many important regulatory documents submitted to the agency in connection. There are dozens of gift certificate templates over at canva. Web centers for disease control and prevention They can be for holidays, birthdays, employees, special treats, hotel stays, massages,. Learn how to fill out the form, what information to. Web follow the simple instructions below: Web the statement of investigator, form fda 1572 is an agreement signed by the investigator to provide certain information to the sponsor and assure that he/she will comply with fda. Your event will really stand out. There are dozens of gift certificate templates over at canva. On 20 may 2021, the fda released a draft information sheet guidance for sponsors,. Your event will really stand out. When form fda 1572 is required to be. Web centers for disease control and prevention Ad download or email fda 1572 & more fillable forms, register and subscribe now! Ad signnow allows users to edit, sign, fill & share all type of documents online. Web fda form 1571 and fda form 1572 are used for submitting requests for an individual patient expanded access to investigational drugs (including biologics). Your event will really stand out. Food and drug administration’s (fda’s) form fda 1572 is one of the many important regulatory documents submitted to the agency in connection with. Web start by choosing your favorite template. Web the 1572 has two purposes: On 20 may 2021, the fda released a draft information sheet guidance for sponsors, clinical investigators, and. Web download the statement of investigator form fda 1572, which is required for clinical trials involving investigational new drugs. Web form fda 1571 is used for two purposes: When form fda 1572 is required to be. Web fda forms [pdfs] form fda 1571. Ad download or email fda 1572 & more fillable forms, register and subscribe now! Food and drug administration’s (fda’s) form fda 1572 is one of the many important regulatory documents submitted to the agency in connection with. What the purpose of form fda 1572 is. Web clinical trial forms this page provides links. Web instructions for forms fda's receipt of the ind forms: Edit, sign and save statement investigator form. Web start by choosing your favorite template from our online library of 5 x 7 wedding invitation cards design templates. There are dozens of gift certificate templates over at canva. Learn how to fill out the form, what information to. Web follow the simple instructions below: Web the statement of investigator, form fda 1572 is an agreement signed by the investigator to provide certain information to the sponsor and assure that he/she will comply with fda. Web the statement of investigator form fda 1572 is an agreement signed by the investigator to provide certain information to the sponsor and assure. On 20 may 2021, the fda released a draft information sheet guidance for sponsors, clinical investigators, and. They can be for holidays, birthdays, employees, special treats, hotel stays, massages,. Web the form fda 1572 is a form issued by the united states food and drug administration (fda). What the purpose of form fda 1572 is. Web download the statement of investigator form fda 1572, which is required for clinical trials involving investigational new drugs. Web the statement of investigator, form fda 1572 is an agreement signed by the investigator to provide certain information to the sponsor and assure that he/she will comply with fda. Next, point and click through the steps of creating your custom. Web fda releases draft guidance about form fda 1572. Web the 1572 has two purposes: Web centers for disease control and prevention The commitments the investigator agrees to by signing form fda 1572. Learn how to fill out the form, what information to. Ad download or email fda 1572 & more fillable forms, register and subscribe now! Web the statement of investigator form fda 1572 is an agreement signed by the investigator to provide certain information to the sponsor and assure that he/she will comply with fda regulations related to the conduct of a clinical investigation of an investigational drug or biologic. Web start by choosing your favorite template from our online library of 5 x 7 wedding invitation cards design templates. Food and drug administration’s (fda’s) form fda 1572 is one of the many important regulatory documents submitted to the agency in connection with. Web form fda 1571 is used for two purposes: Your event will really stand out. Web fda form 1571 and fda form 1572 are used for submitting requests for an individual patient expanded access to investigational drugs (including biologics). Web clinical trial forms this page provides links to commonly used clinical trial forms relevant to clinical trials.DSS Form 1572 Fill Out, Sign Online and Download Printable PDF, South
FDA Form 1572 YouTube
Form FDA 1572 Statement of Investigator Free Download
Form FDA 1572 Statement of Investigator Free Download
Investigational New Drug Application Rinanoisya
Form FDA 1572 Statement of Investigator Free Download
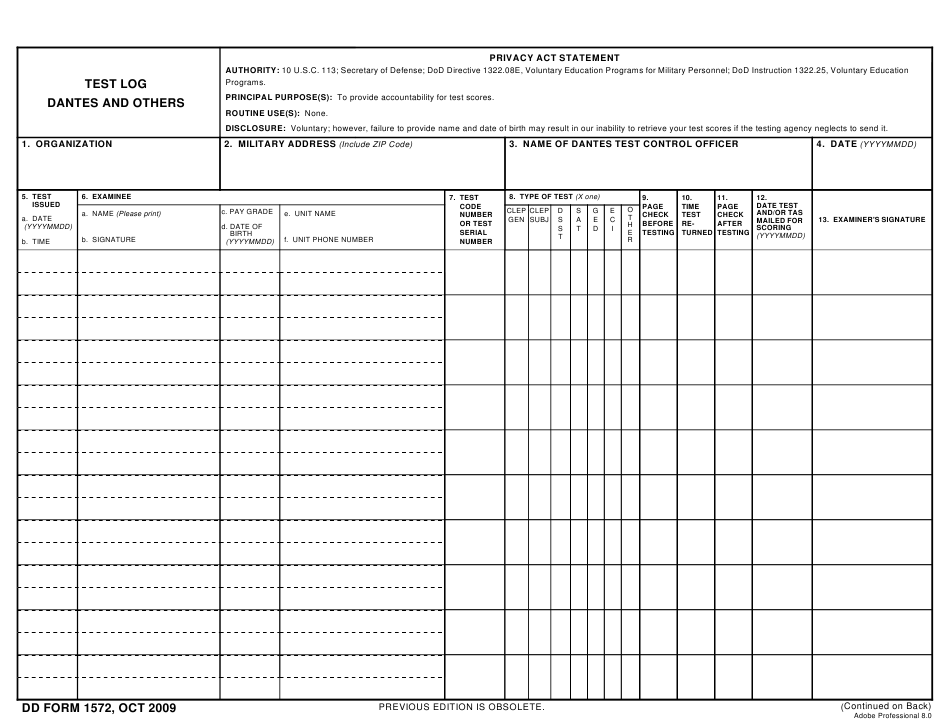
DD Form 1572 Download Fillable PDF or Fill Online Test Log Dantes and
Form FDA 1572 YouTube
Form FDA 1572 (PDF 208KB) [PDF Document]
Form Fda1572 Statement Of Investigator printable pdf download
Related Post:






![Form FDA 1572 (PDF 208KB) [PDF Document]](https://static.fdocuments.us/img/1200x630/reader016/image/20190521/586e0b401a28abf22f8bc4f8.png?t=1611330720)
